Observations of terrestrial biology
- This page is part of the topic Observations, data accuracy and tools
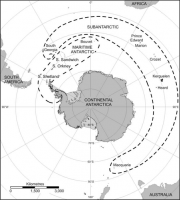
For the purposes of this volume, the Antarctic terrestrial and freshwater biome includes the main continental landmass (the ‘continental Antarctic’ to biologists), the Antarctic Peninsula and associated islands and archipelagos (South Shetland, South Orkney, South Sandwich Islands, Bouvetøya) (the ‘maritime Antarctic’), and the sub-Antarctic islands which lie on or about the Antarctic Polar Frontal Zone (PFZ) (Figure 2.30). These geographic regions are also meaningful biogeographical regions (see Smith, 1984[1]; Chown and Convey, 2006[2], 2007[3]; Huiskes et al., 2006[4]; Convey, 2007b[5])
Over both short and long timescales, there are three major potential colonisation mechanisms likely to have played a role in shaping contemporary Antarctic biodiversity and biogeography, these being simple transport in the air column, incidental transport on other biota and debris, and transport on/in the ocean. Although both oceanic and atmospheric circulation patterns have acted to isolate Antarctica from lower latitudes, at least since the initiation of the Antarctic Circumpolar Current, this barrier is certainly not a hermetic seal, and terrestrial environments of Antarctica and the sub-Antarctic have experienced a fairly constant if low level rate of invasion from temperate or closer regions over evolutionary time, as well acting as a source and exporting biota northwards (Barnes et al., 2006[6]).
Terrestrial and freshwater biological knowledge is unevenly distributed both across these regions and across the different biological groups present (Adams et al., 2006[7]; Chown and Convey, 2007[3]; Peat et al., 2007[8]). Historically, biological research effort has focused on areas easily accessible from research stations, with an understandable but unfortunate tendency to select those areas with obvious biological development. In practice, this means that the majority of biological research (relating to both biodiversity survey and the study of biological adaptation and function) has taken place at a limited number of locations around the continent, and focused on a limited number of organisms. Prime amongst these locations are Signy Island (South Orkney Islands), various locations on the South Shetland Islands, the western coast of the Antarctic Peninsula (Anvers Island, the Argentine Islands, Marguerite Bay), locations along the Victoria Land coastline, and the Victoria Land Dry Valleys. Less accessible and more ‘barren’ areas have historically not received priority for logistic support or science funding, meaning that very little information is available from most inland regions, and many coastal areas and islands remote from research stations. Furthermore, in terms of important but subtle aspects of biodiversity research, in particular the functional role of organisms within an ecosystem, and the provision of ecosystem services, autecological studies of most species in most groups are non-existent (Convey, 1996a[9]; Hogg et al., 2006[10]). This means that functional interpretations of the biology of Antarctic terrestrial biota are often based on (untested) generalisations from the literature on a small number of species that have been targeted, and that on related species and genera from lower latitude ecosystems.
While biodiversity records are obviously available from a much wider range of locations across the continent than the focus areas mentioned, these are often the result of single field campaigns (sometimes directly involving an appropriate specialist, more often opportunistic collections subsequently passed to specialists). It is rare, even where specialists are engaged in field studies, for organised and replicated surveys to be completed. This is owing to multiple reasons - the practical logistics of supporting remote fieldwork, the very patchy distribution and small physical scale of habitats, the typically aggregated distributions of many of the biota involved, and the potential environmental impact and damage caused by sampling sensitive and fragile habitats. Many of the records that do exist are of limited taxonomic usefulness, while even where identifications to species level are available, they often represent the work of a single taxonomist and, especially for the smaller groups of soil invertebrates (e.g. nematodes, tardigrades), have not been re-assessed for veracity since the original collections, in some cases made by the early exploring expeditions (Adams et al., 2006[7]). In many cases, the type material originally described no longer exists or is too degraded to be useful. The contemporary shortage of specialist taxonomic expertise is a problem recognised globally (references from Sands et al., 2008[11]), but is particularly acute with reference to the faunal, floral and microbial groups that constitute Antarctic terrestrial and freshwater ecosystems.
A new tool recently available to Antarctic biologists is that of molecular taxonomy (e.g. Sands et al., 2008[11]). This relies on the use of DNA or RNA sequence substitutions that build up over evolutionary time in order to calculate what are in effect likelihood trees (phylogenetic trees) expressing the evolutionary relationships between different organisms. There are many assumptions inherent in this approach, in particular relating to the rate of substitution over time, how to integrate molecular and classical taxonomic studies, and how to independently ‘ground truth’ the dating of divergence events, but its utility is now generally accepted. Even without attempting to interpret relationships, the use of selected DNA sequences as a molecular ‘barcode’ identifying specific taxa is also becoming widely accepted, and can be seen as an alternative means of assessing biodiversity in the absence of either appropriate taxonomic expertise, or of distinguishing morphological characters.
The limitations of contemporary survey data available as a baseline against which to compare and monitor future trends are amply illustrated by the bryophyte flora, one the best-known and researched groups of Antarctic biota. Peat et al. (2007[8]), based on a comprehensive dataset of confirmed herbarium and literature records, provide a visual illustration and quantification of the level of diversity knowledge of bryophytes across the continent by the simple and coarse means of dividing the continental area in one degree latitude/longitude boxes, identifying all boxes that include at least one ice-free area of > 100 m2, and then identifying how many of these have at least one herbarium or verified literature record of a plant’s occurrence. On this basis, almost exactly 50% of boxes identified have no plant records (although it is then not possible to separate those that have simply not been visited from those that have had any form of visit or survey). Database compilations of diversity for other major groups of Antarctic terrestrial biota are less spatially representative even than that of the bryophytes, but are now becoming available (references in Pugh and Convey, 2008[12]; Convey et al., 2008[13]), starting to generate a baseline against which future changes can be compared.
References
- ↑ Smith, R.I.L. 1984. Terrestrial plant biology of the sub-Antarctic and Antarctic. In: Laws, R.M. (ed.), Antarctic Ecology, 1, Academic Press, London, 61-162.
- ↑ Chown, S.L. and Convey, P. 2006. Biogeography. Trends in Antarctic Terrestrial and Limnetic Ecosystems: Antarctica as a Global Indicator, eds. Bergstrom, D.M, Convey, P. and Huiskes, A.H.L. Springer, Dordrecht, 55-69.
- ↑ 3.0 3.1 Chown, S.L. and Convey, P. 2007. Spatial and temporal variability across life’s hierarchies in the terrestrial Antarctic, Philosophical Transactions of the Royal Society of London, series B, 362, 2307-2331.
- ↑ Huiskes, A.H.L., Convey, P. and Bergstrom, D. 2006. Trends in Antarctic terrestrial and limnetic ecosystems: Antarctica as a global indicator, Trends in Antarctic Terrestrial and Limnetic Ecosystems: Antarctica as a Global Indicator, eds. Bergstrom, D.M, Convey, P. and Huiskes, A.H.L. Springer, Dordrecht, 1-13.
- ↑ Convey, P. 2007b. Influences on and origins of terrestrial biodiversity of the sub-Antarctic islands, Papers and Proceedings of the Royal Society of Tasmania, 141, 83-93.
- ↑ Barnes, D.K., Hodgson, D.A., Convey, P., Allen, C.S. and Clarke, A.C. 2006. Incursion and excursion of Antarctic biota: past, present and future, Global Ecol Biogeogr, 15, 121-142.
- ↑ 7.0 7.1 Adams, B., Bardgett, R.D., Ayres, E., Wall, D.H., Aislabie, J., Bamforth, S., Bargagli, R., Cary, C., Cavacini, P., Connell, L., Convey, P., Fell, J., Frati, F., Hogg, I., Newsham, K.K., O’Donnell, A., Russell, N., Seppelt, R. and Stevens, M.I. 2006. Diversity and Distribution of Victoria Land Biota, Soil Biology and Biochemistry 38, 3003-3018.
- ↑ 8.0 8.1 Peat, H.J., Clarke, A. and Convey, P. 2007 Diversity and biogeography of the Antarctic flora, Journal of Biogeography, 34, 132-146.
- ↑ Convey, P. 1996a. The influence of environmental characteristics on life history attributes of Antarctic terrestrial biota, Biological Reviews of the Cambridge Philosophical Society, 71, 191-225.
- ↑ Hogg, I.D., Cary, S.C., Convey, P., Newsham, K.K., O’Donnell, T., Adams, B.J., Aislabie, J., Frati, F.F., Stevens, M.I. and Wall, D.H, 2006. Biotic interactions in Antarctic terrestrial ecosystems: are they a factor? Soil Biology and Biochemistry, 38, 3035-3040.
- ↑ 11.0 11.1 Sands, C.J., Convey, P., Linse, K. and McInnes, S.J. 2008. Assessing meiofaunal variation among individuals: an example using Tardigrada, BMC Ecology, 8, 7.
- ↑ Pugh, P.J.A. and Convey, P. 2008. Surviving out in the cold: Antarctic endemic invertebrates and their refugia, Journal of Biogeography, 35, 2176-2186.
- ↑ Convey, P., Gibson, J.A.E., Hillenbrand, C-D., Hodgson, D.A., Pugh, P.J.A., Smellie, J.L. and Stevens, M.I. 2008. Antarctic terrestrial life - challenging the history of the frozen continent? Biological Reviews, 83, 103-117.