Understanding the seasonality of Antarctic benthic shelf communities
- This page is part of the topic Marine biology in the instrumental period
The Antarctic spring is one of the planet’s principal episodes of oceanic primary production (Hense et al., 2003[1]), reaching maximum values of 0.1 mg Chl/l in just a few weeks. As more than 107 km2 of sea ice melts, it releases a huge trapped biomass (Thomas and Dieckmann, 2002[2]). Sunlight continues to increase from spring to summer, driving notable changes within an ecosystem just emerging from a long, dark winter. This explosion of life is immediately followed by a growth spurt in the life cycle of the krill, the organism standing at the base of the food chains for nearly all Antarctic marine vertebrates. As winter approaches, the continental shelf and large areas of the open ocean pass back towards a seasonal coverage of ice more than a metre thick, which is why most of the large predators abandon the high Antarctic at the start of the long austral winter.
This pattern led to the conception of a long-lasting paradox – that the ocean around Antarctica experienced pronounced marine seasonality (Clarke, 1988[3]), with a period of low activity in winter as a consequence of reduced food availability, despite the fact that the sea water temperature remained practically constant all year round.
While the marked environmental seasonality naturally does influence and condition life in the water column, the first inklings that the Antarctic paradox might not be entirely accurate arose after the discovery of the rich marine fauna that dwells on the continental shelves in the high Antarctic (Gutt et al., 1992[4]). Over the past twenty years, the region has been shown to host one of the most diverse, high-biomass benthic communities in the ocean (Clarke and Johnston, 2003[5]). Suspension feeders constitute the bulk of these communities, which depend on the particles settling down from the upper layers of the water column or laterally advected to them by currents. Due to low temperatures, a large number of species have slow metabolic rates, associated with a low energy demand, yet they still attain considerable age and size (Peck et al., 2006[6]). This and other traits connected with reproduction patterns would at first glance appear to be in harmony with the tenets of the Antarctic paradox, rooted in the dormant state thought to prevail in winter. However, new features forcing the scientific community to reconsider the Antarctic Paradox have recently come to light. For instance, quite a few species exhibit reproduction rates similar to those in other regions, while others demonstrate higher growth rates than expected by quickly occupying areas scraped clean by icebergs (Teixidó et al., 2004[7]). Experimental observations have furnished earlier selected results (Barnes and Clarke, 1994[8], 1995[9]) supporting the assumption that the long Antarctic winter may not be as inactive as hitherto thought. These findings include:
- The existence of “food banks” extending over hundreds of kilometres, offering a potential food source for numerous bottom-dwelling organisms (Mincks et al., 2005[10]). This phenomenon also known as “green carpets” tends to form at the beginning of the austral spring, when the high primary production generated by melting ice is not immediately exploited by planktonic grazers and settles on the shelf seabed in a time span of hours to days (Gutt et al., 1998[11]).
- Widespread distribution of seabed sediment with high nutritive quality and grain sizes suitable for the anatomic structures of benthic suspension feeders. On average, measured concentrations of protein (3 mg/g) and lipids (2 mg/g) are higher than on other continental shelves and similar to the contents found in settling particles (Smith et al., 2006[12]).
- Tides acting as an incessant mechanism to resuspend the “food banks” and supply particles to suspension feeders throughout the year (Smith et al., 2006[12]).
- Benthic suspension feeders on Antarctic shelves feeding on small-sized particles in contrast to species from other latitudes that mainly ingest zooplankton (Orejas et al., 2003[13]).
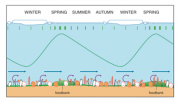
The new evidence of the physical-chemical conditions on the shelf seabed at Antarctic latitudes makes it necessary to reconsider the paradox that had formerly served as a cornerstone for understanding polar ecosystems. After the summer, resuspension by tidal currents and the high nutritional quality of the seabed sediment allow benthic trophic conditions to remain almost constant throughout the year, which provides the basis for a new model of Antarctic seasonality. The new model helps to explain the high diversity and high biomass of benthic communities around Antarctica, even when the food input from the euphotic zone becomes scarce when ice covers the ocean surface during the winter months. These new findings must be taken into account when planning future research on the Antarctic bottom-dwelling fauna. Special emphasis should be placed on carrying out studies during the austral winter, when processes occurring near the seabed (Figure 4.52) could be a key to understanding both the high productivity of the system in the early spring and the high biodiversity of the benthic ecosystem (Gili et al., 2006[14]).
References
- ↑ Hense, I., Timmermann, R., Beckmann, A.and Athmann, U.V. 2003. Regional and interannual variability of ecosystem dynamics in the Southern Ocean, Ocean Dynamics, 53, 1-10.
- ↑ Thomas, D.N. and Dieckmann, G.S. 2002. Antarctic sea ice-a habitat for extremophiles, Science, 295, 641-644.
- ↑ Clarke, A. 1988. Seasonality in the Antarctic marine environment, Comp. Biochem. Physiol., 90(B), 461-473
- ↑ Gutt, J., Gerdes, D. and Klages, M. 1992. Seasonality and spatial variability in the reproduction of two Antarctic holothurians (Echinodermata), Polar Biol., 11, 533-544.
- ↑ Clarke, A. and Johnston, N.M. 2003. Antarctic marine benthic diversity, Oceanogr. Mar. Biol. Ann. Rev., 41, 47-114.
- ↑ Peck, L.S., Convey, P. and Barnes, D.K.A. 2006. Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability, Biol. Rev., 81, 75-109.
- ↑ Teixidó, N., Garrabou, J., Gutt, J. and Arntz, W.E. 2004. Recovery in Antarctic benthos after iceberg disturbance: trends in benthic composition, abundance and growth forms, Mar. Ecol. Prog. Ser., 278, 1-16.
- ↑ Barnes, D.K.A. and Clarke, A. 1994. Seasonal variation in the feeding activity of four species of Antarctic bryozoa in relation to environmental factors, J. Exp. Mar. Biol. Ecol., 181, 117-133.
- ↑ Barnes, D.K.A. and Clarke, A. 1995. Seasonality of feeding activity in Antarctic suspension feeders. Polar Biol., 15, 335-340.
- ↑ Mincks, S.L., Smith, C.R. and Demaster, D.J. 2005. Persistence of labile organic matter and microbioal biomass in Antarctic shelf sediments: evidence of a sediment ‘food bank’, Mar. Ecol. Prog. Ser., 300, 3-19.
- ↑ Gutt, J., Starmans, A. and Dieckmann, G. 1998. Phytodetritus deposited on the Antarctic shelf and upper slope: its relevance for the benthic system, J. Mar. Syst., 17, 435-444.
- ↑ 12.0 12.1 Smith, C.R., Minks, S. and Demaster, D.J. 2006. A síntesis of bentho-pelagic coupling on the Antarctic shelf: Food banks, ecosystem inertia and global climate change, Deep-Sea Res. II, 53, 875-894.
- ↑ Orejas, C., Gili, J-M. and Arntz, W. 2003. Role of small-plankton communities in the diet of two Antarctic octocorals (Primnoisis antarctica and Primnoella sp.), Mar. Ecol. Prog. Ser., 250, 105-116.
- ↑ Gili, J-M., Arntz, W.E., Palanques, A., Orejas, C., Clarke, A., Dayton, P., Isla, E., Teixidó, Rossi, S. and López-Gonzales, P.J. 2006. A unique assemblage of epibenthic sessile suspension feeders with archaic features in the high-Antarctic, Deep-Sea Res. II, 53, 1029-1052.