Difference between revisions of "Near-shore marine disturbances over the next 100 years"
(Changed Figure 5.15 to link to page) |
(Changed book section reference to page link) |
||
| Line 104: | Line 104: | ||
About half of the anthropogenically emitted CO<sub>2</sub> has been absorbed by the global ocean (Key et al., 2004<ref name="Key et al, 2004">Key, R.M., Kozyr, A., Sabinec, L., Lee, K., Wanninkhof, R., Bullister, J., Feely, R.A., Millero, F., Mordy, C. and Peng, T-H. 2004. A global ocean carbon climatology: results from GLODAP, ''Global Biogeochemical Cycles'', '''18''', GB4031</ref>), making the global surface ocean more acid by on average 0.1 pH units. This seems like a small amount, but it is equivalent to a 30% increase in hydrogen ions because the pH scale is logarithmic. In both ecological and evolutionary contexts this constitutes a major disturbance. Orr et al. (2005<ref name="Orr et al, 2005">Orr, J.C., Fabry, V.J., Aumont, O., Bopp, L., Doney, S.C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R.M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R.G., Plattner, G.K., Rodgers, K.B., Sabine, C.L., Sarmiento, J.L., Schlitzer, R., Slater, R.D., Totterdell, I.J., Weirig, M.F., Yamanaka, Y. and Yool, A. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms, ''Nature'', '''437''', 681-686.</ref>) demonstrate that the rate of acidification will likely increase over the next few centuries, and that acidification will penetrate progressively deeper into the ocean for centuries after fossil fuels have been used up; surface ocean pH levels may become more acid by 0.2 to 0.3 units by 2100. Thus the magnitude of acidification will increase as an agent of disturbance both in absolute terms and spatially. Because levels and rates of acidification are greatest at the surface, organisms in the shallows and on the continental shelf will be influenced first and more seriously – this is likely to be a major problem for animals that secrete CaCO<sub>3</sub> skeletons. Nevertheless it should be borne in mind that pre-industrial ocean pH was around 8.2, which is slightly alkaline compared with that of neutral water (pH 7). So the current changes to pH levels of 8.1 and towards 8.0 or 7.9 could be said to be making the ocean less alkaline rather than actually acid (acids have pH<7). | About half of the anthropogenically emitted CO<sub>2</sub> has been absorbed by the global ocean (Key et al., 2004<ref name="Key et al, 2004">Key, R.M., Kozyr, A., Sabinec, L., Lee, K., Wanninkhof, R., Bullister, J., Feely, R.A., Millero, F., Mordy, C. and Peng, T-H. 2004. A global ocean carbon climatology: results from GLODAP, ''Global Biogeochemical Cycles'', '''18''', GB4031</ref>), making the global surface ocean more acid by on average 0.1 pH units. This seems like a small amount, but it is equivalent to a 30% increase in hydrogen ions because the pH scale is logarithmic. In both ecological and evolutionary contexts this constitutes a major disturbance. Orr et al. (2005<ref name="Orr et al, 2005">Orr, J.C., Fabry, V.J., Aumont, O., Bopp, L., Doney, S.C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R.M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R.G., Plattner, G.K., Rodgers, K.B., Sabine, C.L., Sarmiento, J.L., Schlitzer, R., Slater, R.D., Totterdell, I.J., Weirig, M.F., Yamanaka, Y. and Yool, A. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms, ''Nature'', '''437''', 681-686.</ref>) demonstrate that the rate of acidification will likely increase over the next few centuries, and that acidification will penetrate progressively deeper into the ocean for centuries after fossil fuels have been used up; surface ocean pH levels may become more acid by 0.2 to 0.3 units by 2100. Thus the magnitude of acidification will increase as an agent of disturbance both in absolute terms and spatially. Because levels and rates of acidification are greatest at the surface, organisms in the shallows and on the continental shelf will be influenced first and more seriously – this is likely to be a major problem for animals that secrete CaCO<sub>3</sub> skeletons. Nevertheless it should be borne in mind that pre-industrial ocean pH was around 8.2, which is slightly alkaline compared with that of neutral water (pH 7). So the current changes to pH levels of 8.1 and towards 8.0 or 7.9 could be said to be making the ocean less alkaline rather than actually acid (acids have pH<7). | ||
| − | As discussed | + | As discussed in [[The Southern Ocean carbon cycle response to future climate change#Response to increased CO2 uptake|The Southern Ocean carbon cycle response to increased CO2 uptake]], under normal conditions calcite and aragonite are stable in surface waters because the carbonate ion is supersaturated there. As pH falls, so does the concentration of the carbonate ion. If it falls to the point that carbonate becomes under-saturated, then structures made of calcite or aragonite may begin to dissolve. Because the Southern Ocean has low saturation levels of CaCO<sub>3</sub>, and because it is the location of much of the uptake of carbon dioxide from the atmosphere, it is at more risk than other areas of approaching under-saturation, particularly for animals that use the aragonite form of CaCO<sub>3</sub>. Model projections of changing oceanic pH show that Southern Ocean saturation levels could become critical for organisms using aragonite within the next 100 years (Orr et al., 2005<ref name="Orr et al, 2005">Orr, J.C., Fabry, V.J., Aumont, O., Bopp, L., Doney, S.C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R.M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R.G., Plattner, G.K., Rodgers, K.B., Sabine, C.L., Sarmiento, J.L., Schlitzer, R., Slater, R.D., Totterdell, I.J., Weirig, M.F., Yamanaka, Y. and Yool, A. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms, ''Nature'', '''437''', 681-686.</ref>). Increased difficulty in synthesising skeletal material could pose major problems for the survival of many Southern Ocean species, particularly the aragonitic pteropods that are an important part of the plankton at the base of the food chain. Despite these fears there is evidence from the North Atlantic of increases in calcification of coccolithophores (calcitic plankton) over the past 200 years at the same time that the ocean has become less alkaline (Iglesias-Rodriguez et al., 2008<ref name="Iglesias-Rodriguez et al, 2008">Iglesias-Rodriguez, M.D., Halloran, P.R., Rickaby, R.E.M., Hall, I.R., Colmenero-Hidalgo, E., Gittins, J.R., Green, D.R.H., Tyrrell, T., Gibbs, S.J., Von Dassow, P., Rehm, E., Armbrust, E.V. and Boessenkool, K.P. 2008. Phytoplankton Calcification in a High-CO<sub>2</sub> World. Science, 320, 336-340. doi:10.1126/science.1154122.</ref>). Bearing this conflicting evidence in mind, the jury remains out for the moment as far as the current effects of ocean acidification are concerned, though there is a worry about what the effect of the further predicted pH changes may be. |
==Deoxygenation== | ==Deoxygenation== | ||
Latest revision as of 17:49, 22 August 2014
- This page is part of the topic Marine biology over the next 100 years
Contents
- 1 Introduction
- 2 Rapid ice-loading increases and decreases from episodic scour by icebergs
- 3 Coastal sedimentation from glaciers and due to deglaciation on land
- 4 Freshening events, from increased seasonal melting of glaciers, ice sheets and ice shelves
- 5 Short-term thermal events and natural oscillations such as El Niño events
- 6 Ice shelf disintegration exposing new habitat
- 7 Long-term ice scour decreases from a decreasing supply of icebergs
- 8 Warming at the whole Southern Ocean scale
- 9 Benthic response to changes in pelagic systems
- 10 Acidification
- 11 Deoxygenation
- 12 References
Introduction
The Southern Ocean has the highest wind speeds and wave heights and the most icebergs of any water body. Since the formation of a massive ice sheet over the southern polar region, the biodiversity on the shelf of the Southern Ocean has been exposed to significant disturbance, mainly from scouring by the keels of moving icebergs and ice shelves that have sculpted the seabed and disturbed the benthic biota. As a result, the shelf environments of the Antarctic are amongst the most disturbed places on the planet, especially considering the slow tempo of polar coldblooded animals (ectotherms) and their inability to recolonise rapidly (Gutt and Starmans, 2001[1]). In contrast the Southern Ocean also includes some of the world’s least disturbed seabeds, both in the abyss and beneath the marginal ice shelves year round, and seasonally on the shelf below winter sea ice. Both in the shallows (Smale et al., 2007[2]) and on the shelf down to depths of a few hundred metres (Gutt and Piepenburg, 2003[3]) the seabed resembles a patchwork quilt of recovery since the last iceberg scour events. Despite the shallower parts of the continental shelf being very disturbed, they have the highest regional biodiversity as a result of continual processes of recolonisation (Gutt and Piepenburg, 2003[3]). Newly scoured areas are dominated by rapidly spreading and rapidly growing pioneers, whereas less affected areas are occupied by slower growing, more competitively dominant species. Polar benthic communities can be highly hierarchical. Where there is no mechanical disturbance the major space competitors can monopolise space virtually unchallenged, producing a monoculture, as found mainly on the shallower parts of the shelf. Future changes in disturbance are likely to be highly variable regionally, in line with the regional development of warming. For example, we should expect considerable and continuing change in disturbance in the Antarctic Peninsula region, the area where the greatest warming and ice retreat is taking place (Cook et al., 2005[4]).
Models of how the Southern Ocean will respond physically to the drastic and unprecedented, recent, rapid rises in CO2 and temperature have high associated levels of error. Nevertheless, as shown above, extreme temperatures are not expected; models suggest a 0.5º to 1.0ºC rise in the temperature of Southern Ocean surface waters in summer over the next 100 years, possibly increasing to 1.5ºC in the Amundsen Sea, while winter SSTs remain within 0.5ºC of where they are now, except locally where they may rise by 1.0ºC. The temperatures on the shelf at depths of 200 m are likely to be much the same as those at the surface.
As well as warming, other physical responses in the Southern Ocean have already been detected including shrinking, duration and extent of seasonal sea ice (Zwally et al., 2002a[5]), glacial retreat (Cook et al., 2005[4]), rising acidification, and desaturation of aragonite levels (Key et al., 2004[6]). Sea ice is expected to decrease by 33% overall, but with considerable regional variation, declining most in the Weddell Sea in December through May, and in the Amundsen and Bellingshausen Seas and in the South Atlantic and south Indian Ocean sectors in June through November (Figure 5.17). The gradual disappearance of ice shelves will open up new areas of ocean and seabed as habitat and for primary production.
Changes in disturbance with regional warming can be classed as acute (rapid change on short ecological time scales) or chronic (change on time scales spanning hundreds of years). Major elements of disturbance envisaged to alter with continued regional warming include:
Acute:
- Rapid ice-loading increases and decreases, caused by a rise in episodic scour by icebergs
- Coastal sedimentation, caused by a rise in discharge from glaciers due to deglaciation on land
- Freshening events, caused by increased seasonal melting of glaciers, ice sheets and ice shelves
- Thermal ‘events’, caused by natural oscillations like El Niño events
Chronic:
- Ice shelf disintegration, exposing new habitat
- Long term ice scour decreases, from a decreasing supply of icebergs
- Warming, at the whole Southern Ocean scale
- Benthic response to changes in pelagic systems (e.g. decreasing the supply of food to the seabed)
- Acidification
- Deoxygenation
In the following subsections, the way in which each of these disturbance events may develop is considered as a ‘best guess’. Acute events are considered first because they most easily conform to normal definitions of disturbance, but on evolutionary time scales chronic events may have just as much or more impact.
Rapid ice-loading increases and decreases from episodic scour by icebergs
Currently we are in an interglacial period and so should expect that some of the observed glacier and ice shelf retreat may be a continued response to the warming following the glacial maximum. There is evidence that a number of today’s ice shelves were not present during the last interglacial, when it was warmer than now. Nevertheless, recent glacier retreat and ice shelf collapse in some regions, such as the Antarctic Peninsula, is almost certainly occurring faster than would be expected during natural cycles, whilst other regions have experienced either no change or small increases in ice cover. Many retreating maritime glaciers and ice shelf fronts have not yet retreated past their grounding lines and therefore calving ice will, at least over short timescales, continue to generate floating icebergs.
Ice shelves hold glaciers in check, so the disintegration of ice shelves adds to the production of icebergs and associated scour in two ways – first as chunks of ice shelf, and second from disintegration of the accelerated glaciers that were formerly held in check. An increased ‘population’ of Southern Ocean icebergs would lead to more ice scouring, with severe implications for shelf benthos. Although Antarctic icebergs can have draughts of up to 600 m depth, exposing most of the Antarctic shelf system to scour, most have draughts of 250 m or less, which generally restricts scour to the upper continental shelf.
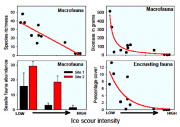

Iceberg groundings are catastrophic and cause high mortality to benthic assemblages at scales of tens to hundreds of square metres, as in shallow waters around the South Orkneys, for example, where Peck et al. (1999[7]) observed a 99.5% reduction in macrofaunal abundance following iceberg scour. Similar decreases have been found near the Antarctic Peninsula (Figure 5.26, Figure 5.27). As a consequence, both in the shallows (Smale et al., 2007[2]) and on the shelf down to depths of a few hundred metres (Gutt and Piepenburg, 2003[3]) the seabed resembles a patchwork quilt of recovery since the last iceberg scour events. Despite the shallower parts of the continental shelf being very disturbed, they have the highest regional biodiversity as a result of continual processes of recolonisation. Newly scoured areas are dominated by rapidly spreading and rapidly growing pioneers, whereas less affected areas are occupied by slower growing, more dominant species. At greater depths it seems that iceberg scouring promotes and maintains biodiversity by increasing habitat heterogeneity and preventing monopolisation by dominant competitors (Gutt and Starmans, 2001[1]). Polar benthic communities can be highly hierarchical. Where there is no mechanical disturbance the major space competitors can monopolise space virtually unchallenged, producing a monoculture, as found mainly on the shallower parts of the shelf. Because iceberg scour damages benthos, it promotes feeding opportunities for scavengers, which may be abundant at chronically disturbed locations. An enhancement to ice scouring in coastal waters will lead to benthic communities becoming dominated by pioneers (r strategists) and scavengers, whilst the species richness and the abundance of large and longer lived species will decline.
Increased iceberg scouring will also reduce the local densities and biodiversity of the meiofauna at an initial stage. Densities may recover quickly during recolonisation, but the community will remain impoverished for a long time after disturbance, especially at deeper shelf areas. The communities from shallow sub-tidal areas regularly affected by iceberg scouring seem better adapted to this process of physical disturbance (Lee et al., 2001a[8],b[9])
It seems likely that the numbers of icebergs will increase over the next hundred years or so, but the pattern of their seasonal and regional distribution may change with the projected reduction in sea ice cover. For example, we should expect considerable and continuing change in disturbance in the Antarctic Peninsula region, the area where the greatest warming and ice retreat is taking place (Cook et al., 2005[4]). It is well known that the frequency of ice scouring in coastal waters is strongly linked to the persistence of seasonal fast ice (Smale et al., 2007[2]). During the winter months icebergs are ‘locked in’ by fast ice (sea ice that is locked to the coast and lasts more than one year) that has formed around them, which restricts their movements and therefore their potential to cause disturbance. The extent and duration of fast ice has decreased in some regions during recent decades (Zwally et al., 2002a[5]), and further reductions in sea ice duration would mean that icebergs will be more free to move around coastal waters for longer periods of the year. Thus, we foresee an increase in both ice scouring frequency and duration in coastal waters.
Coastal sedimentation from glaciers and due to deglaciation on land
Many maritime glaciers have dramatically retreated in recent decades along the Antarctic Peninsula, and the flow of ice into coastal waters is accelerating there and in the Bellingshausen Sea. The increased sedimentation associated with these glacial retreats is likely to have a considerable, but localised, effect on benthic communities adjacent to glacial termini. For example, a retreating Alaskan glacier may deposit up to 14 cm of sediment annually at its terminus (Cowan et al., 2006[10]), whilst acute ice calving events considerably increase water column turbidity, and rapid glacier surges can lead to a 30-fold increase in seabed sedimentation for 4 km from the ice front (Gilbert et al., 2002[11]). Directly beneath retreating ice fronts, where sedimentation rates are greatest, benthic fauna are completely smothered and the seabed is largely inhospitable. Studies on the effects of sedimentation on polar coastal benthos have been conducted almost exclusively at Spitsbergen in the Arctic, but the physical processes driving the observed patterns are likely to be consistent at both poles, despite considerable differences in the assemblages concerned.
In the most comprehensive study of its kind, Syvitski et al. (1989[12]) sampled the benthos inhabiting ten Arctic fjords influenced by glaciers at differing stages of retreat. They proposed a general model of benthic community change during glacier retreat, which was principally driven by sedimentation rates. The seabed proximal to a retreating glacier is characterised by exceptionally high sedimentation and supports a pioneer assemblage of very few species (perhaps just one) of macrobenthic deposit feeders. This simple assemblage is likely to persist until the glacier front has retreated onto land, at which point sedimentation decreases to a moderate intensity and a more complex assemblage, still largely devoid of suspension feeders, can develop. The final stage in the faunal succession can occur once a glacier has retreated across land to expose an extensive valley floor, which filters sediment discharge and restricts the transport of sediment to the sea. The reduced sedimentation allows greater light penetration and causes minimal smothering, so that the seabed supports a diverse community, including suspension feeders and predators. This model has been supported by studies elsewhere, and Antarctic communities inhabiting the shelf adjacent to retreating glacier fronts might undergo similar change over ecological timescales. If, however, deglaciation continues on land and, consequently, increasing amounts of suspension are washed into the ocean by precipitation, as recently observed east of the Antarctic Peninsula (Figure 5.28), the benthos might even become poorer compared to a former ice shelf edge, and filter feeders will not recover not only at the former glacier termini but over large coastal areas. Instead only a reduced number of opportunistic deposit feeders or infauna would survive and perhaps benefit, and diversity would remain low.
Glacial retreat is likely to increase sediment loading in the shallow parts of steep sections of shelf, and could destabilise the substrate and promote slumping events. Such events could disturb assemblages over a considerable area, perhaps to depths of the order of hundreds of metres. Slumping of unstable sediments may smother considerable areas inhabited by benthos, and can result in high mortality of sessile species, reduced richness and significant community restructuring (see e.g. Gambi and Bussotti, 1999[13]). Retreating ice fronts also deposit quite coarse material including boulders and ‘drop stones’. Although small scale smothering by drop stones will damage sessile benthos, drop stones also represent an important source of hard substratum for colonisation.
Freshening events, from increased seasonal melting of glaciers, ice sheets and ice shelves
Compared with the Arctic (and lower latitude coastal environments) the influence of freshwater on marine benthos in Antarctica is minimal. Even so, during Antarctic summers melt water dilutes surface seawater (to depths of ~10 m), and is an important stressor on very shallow water benthos and intertidal biota (<10 m deep). The coastal waters of some Antarctic regions are likely to experience a considerable increase in freshwater input over the next hundred years from glacial retreat and melting ice sheets. IPCC model data (Figure 5.15(ii) on Ocean change over the next 100 years) suggest that sea surface salinity around Antarctica will freshen by 0.1 to 0.2 units, with local values up to 0.3 in the Weddell Sea, in the Ross Sea, off Oates Land and in a few patches elsewhere along the coast; the winter pattern is similar to the summer pattern, but may freshen up to 0.3 units west of the Antarctic Peninsula. The model data show that these changes are restricted to surface waters. Stockton (1984[14]) observed mass mortality in an epifaunal bivalve population following the summer formation of relatively fresh seawater at McMurdo Sound, but this is the only field report of its kind. Conversely, in the laboratory some (mostly) intertidal Antarctic algae exhibited rather broad salinity tolerances between 7 and 68 units for growth, photosynthesis and respiration (Wiencke et al., 2007[15]). Both the intensity and frequency of biologically important freshening events are likely to increase in some regions in response to continued warming. Indeed, it is known that the salinity of the surface waters of the Ross Sea decreased during the late Twentieth Century, probably as a result of increased precipitation, reduced sea ice formation and continued melting of the West Antarctic ice sheet in response to global warming (Jacobs et al., 2002[16]). Surface freshening on this scale can have a wide range of effects on both the water column and the seabed below it, including increased stratification and therefore reduced penetration of light and oxygen through the water column; these changes as well as the changes in salinity will have deleterious biological effects. Nevertheless it should be borne in mind that the modelled changes appear relatively small.
Short-term thermal events and natural oscillations such as El Niño events
Short periods of cold, such as ‘ice winters’ in temperate Europe, or unusually warm conditions in the tropics (associated with stronger El Niño Southern Oscillation (ENSO) events) have famously caused widespread mortality in the shallows. In the Antarctic, explosive growth of the exceptionally fast growing sponge Homaxinella balfourensis in McMurdo Sound has been related to the 1982/1983 El Niño event (Dayton, 1989[17]). Even so, major thermal events and responses, are not common in the Southern Ocean. In winter that is because winter sea temperatures are already at their minimum, and because extensive vertical mixing induced by strong winds and waves limits any rise in sea temperatures in the shallows. The sole exception is close to active volcanoes, as at Deception Island. Most research into potential responses of biodiversity to climate change in Antarctica as elsewhere, have been dominated by considering the effects, in aquaria, of acute warming in very rapid rises (≥0.5°C per day) or rapid rises (e.g.1-3°C per week). We have already discussed these results, above. To date these experiments have largely been conducted on relatively few and arguably atypical species, the common and abundant types in the shallows, so how well these typify Antarctic biodiversity is unknown. There is also debate about the extent to which such short-term experiments and rapid temperature rises reflect true vulnerability to chronic regional warming, although certainly they do suggest the fauna is highly sensitive to acute warming. Current climate models suggest that acute warming is unlikely, as are average rises of more than 1.5ºC (Figure 5.15 on Ocean change over the next 100 years).
Ice shelf disintegration exposing new habitat
Life below ice shelves is common in the Antarctic, since one third of the Antarctic continental shelf is covered by floating ice shelves. Due to their inaccessibility, the habitats under ice shelves belong to the least known on Earth. With an almost overnight ice shelf collapse, the change from an extremely oligotrophic ice shelf-covered ecosystem to a normal Antarctic shelf ecosystem, with high primary production during a short summer, is likely to be one of the largest ecosystem changes on the planet. First investigations in the Larsen B area following the collapse of that ice shelf showed that unique benthic species assemblages adapted to specific trophic conditions that might vanish (Figure 5.29) Krill (Euphausia superba and E. crystallorophias) and the pelagic fish Pleuragramma antcticum, appeared 5 to 12 years after the area became available, followed by seals (mainly Crabeaters), and Minke Whales (Gutt et al., 2008[18]). In contrast, penguins, which inhabit rookeries about 60-100 km north of Larsen B had not yet entered the area. The sea-floor was dominated by very few fast growing species such as ascidians (Figure 5.30), sea-urchins with pelagic larvae, and young glass sponges.
Concerning the meiofauna, the area closest to the open Weddell Sea is characterized by much higher densities and percentages of nematodes than are found in the formerly ice shelf-covered bays. The outer stations might have close to a climax community, dominated by nematodes, whereas communities at the inner stations might have been sampled at an intermediate stage of succession, with lower densities but higher diversity.
Long-term ice scour decreases from a decreasing supply of icebergs
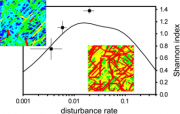
Whilst over short timescales (i.e. decades) it seems likely that ice loading and associated ice scour will increase in coastal waters, over longer timescales (i.e. centuries) it is likely that the frequency of ice scouring will diminish. Currently, about 55% of the seaward margin of the Antarctic Ice Sheet comprises floating ice, but this proportion will decrease as more ice shelves collapse. Once maritime glaciers and ice shelf fronts retreat onto land and past their grounding lines, calving ice will remain landlocked rather than being deposited into coastal waters where it can disturb marine benthos. Similarly, although the disintegration of an ice shelf can lead to rapid coastal ice loading by accelerating ice flows (Scambos et al., 2004[21]), this can only be sustained for a finite period. So, whilst the major Antarctic ice streams will continue to transfer ice from land to sea over long time scales, the rate of ice deposition by small maritime glaciers and ice shelves will almost certainly reduce over time. The maximum depth at which ice scouring can occur is also likely to decrease, as the predicted retreat of the Antarctic ice sheet will result in thinner ice shelves and thus thinner calving icebergs. The current maximum draught of large tabular icebergs is rarely more than 600 m (Dowdeswell and Bamber, 2007[22]). Relict scour marks, from when polar ice sheets were more extensive, have been detected in much deeper waters. If icebergs in the future have smaller draughts, the marine benthos inhabiting the deeper waters of the shelf will become unaffected by ice disturbance, so may become less diverse. Johst et al. (2006[19]), using the Weddell Sea shelf with an average disturbance regime as a model system, found that a decrease in disturbance frequency was likely to be more detrimental to regional biodiversity than is a moderate increase (Figure 5.31).
It is likely that disturbance regimes (and therefore community structure) in the shallowest waters will experience the greatest change. The shallow subtidal and intertidal zones around Antarctica are currently amongst the most frequently disturbed habitats on Earth. During summer, small icebergs and rafts of sea ice constantly scrape the shallow sea floor. In contrast, during winter, the icefoot - a narrow fringe of ice attached to the coastline - envelopes the seabed and associated fauna. As a result of intense disturbance, these habitats generally (but not always) support very simple assemblages, often dominated by opportunistic pioneers or mobile species that can retreat to deeper waters during winter. It seems likely that scouring by sea ice rafts and disturbance by the winter ice foot in shallow waters will eventually decrease with continued climate warming, as sea ice and ice foot formation and longevity will diminish under warmer conditions. The responses of shallow water and intertidal benthic communities to decreasing disturbance frequencies are difficult to predict, but it is evident that current disturbance holds community development at early successional stages in these habitats.
Warming at the whole Southern Ocean scale
Across much of the planet sea surface temperatures are warming but increases are much greater in some areas than others (Hansen et al., 2006[23]). Although warming is difficult to detect in the surface layers of the Southern Ocean, due to a significant seasonal cycle and only few data, a warming signal of 2.3°C in August over the past 81 years, with about half the intensity in summer, is clearly visible in the upper 150 m around South Georgia (Whitehouse et al., 2008[24]). In addition the surface of the Bellingshausen Sea has warmed rapidly by 1ºC over the last 50 years, but this signal rapidly diminishes away from the top hundred metres of the water column (Meredith and King, 2005[25]). In intermediate layers (300 – 1000 m) warming by up to 0.3°C over almost 50 years (Böning et al., 2008[26]) is significant but clearly smaller. Model outputs suggest that more widespread warming is inevitable, but that the most likely maxima are 0.5 to 1.0ºC, only locally reaching 1.5ºC (see Figure 5.15 on Ocean change over the next 100 years).
Whether populations or species will survive future temperature rises may be dictated by their ability to do critical activities such as feeding. Much of the evidence for the possible response to warming is based on laboratory experiments, which can only be performed on ecologically short timescales. That begs the question “Does the level of sensitivity exhibited in these short term experiments represent true vulnerability to likely environmental change, which will occur at a much slower rate?” Furthermore, in the natural environment sea temperatures vary with time, especially seasonally, and are only at maximum levels for a month or so – rather than continuous as in experiments, which leads to further doubt as to the meaning of the experiments. There are also questions as to how important the ‘critical activities’ measured to date in experiments are to survival, and how representative the species tested to date are of the wide biodiversity present. For example, the species that are convenient to sample and use as models, those in the shallows, are often atypical of their taxa in being highly dispersive (Poulin et al., 2002[27]). Many other Antarctic taxa have current distributions that include sites or depths encompassing a much greater temperature range than ‘typical Antarctic conditions’. South Georgia, for example, has populations of many otherwise typical Antarctic species, despite experiencing maximum summer temperatures 3°C warmer than some localities on the western side of the Antarctic Peninsula (Barnes et al., 2006b[28]). Although Antarctic species tend to be endemic, in many taxa up to 40% of the species have ranges stretching into temperate waters where seasonal minimum temperatures exceed maximum values in the Southern Ocean. Such evidence implies that there may be a conflict between physiological (i.e. experimental) and ecological evaluations of vulnerability; ecological context may be a key missing ingredient. To gain more meaningful estimates of true vulnerability will require, for instance, investigations of populations across latitudes and depths, and at sites with differing temperature regimes, along with assessments of community level responses to changing conditions. This explains why biological response to predicted temperature rises in the Southern Ocean over the next 100 years is unclear despite experimental evidence indicating high levels of sensitivity.
Benthic response to changes in pelagic systems
The macrobenthos depends on a broad variety of food sources: suspended and deposited phytodetritus, other organic particles such as faecal pellets and aggregates, animals, macroalgae, and carrion. For the major primary food source, phytodetritus, it is possible to predict both an increase in supply due to an extended period for algal blooms in open water following a reduction in sea ice cover, and a reduction (see Thrush et al., 2006[29]), at least at the regional level, due to there being less sea ice to melt and, as a consequence, a less well developed halocline.
It seems likely that most shelf-inhabiting suspension feeders may be adapted to the limited food supply typical of recent past glacial periods (Gutt, 2000[30]). A further climate-induced increase in food supply could cause some species to suffer from a clogging of their feeding apparatus. The demise of such suspension feeders would also have potential consequences for the rich associated fauna, since their microhabitat or food source will be lost. Similar negative effects must be expected for large-scale geo-engineering operations to sequester anthropogenic CO2 by fertilizing the Southern Ocean with iron (Smith et al., 2008[31]). In contrast to ecological theory, fertilized systems with increased food supply are usually characterised by an increasing dominance of a few species only, and extinction of the majority of species (for examples from the deep-sea see Billett et al., 2001[32]; Schewe and Soltwedel, 2003[33]). As a consequence, in the Antarctic the extremely species-rich deep-sea fauna (Brandt et al., 2007[34]), roughly estimated as 100 times richer than that of the Antarctic shelf, would be endangered. Since all deep-sea basins in the Southern Hemisphere are connected by the Southern Ocean, such negative effects may become widespread.
Due to fertilisation of surface waters by the upwelling of warm Circumpolar Deep Water over the Antarctic continental slope, increased sedimentation rates of phytodetritus may be expected anywhere in the Southern Ocean, and may have a similarly deleterious effect on the diversity of the benthic biota, even on the shelf. While Antarctic surface waters are zoogeographically isolated by the Polar Front, the deep-sea fauna is connected to the Antarctic slope and shelf fauna. Consequently, what happens on the slope and shelf could also affect the deep-sea part of the Antarctica’s unique fauna and flora.
Only benthic systems living under strongly limiting food conditions (see Post et al., 2007[35]; Riddle et al., 2007[36]) might become richer in diversity and biomass, when food conditions improve, e.g. in areas where ice shelves disintegrate. An assemblage well adapted to extremely poor food conditions might suffer a decline rather than increase in diversity if the supply of food increased.
If the food supply reduces, Antarctic suspension feeders are generally not expected to suffer since they are able to survive extended periods of time (months to years) without food. Only communities that experience dramatic changes in local current conditions, e.g. due to a change in the shape of the ice shelf edge (Seiler and Gutt, 2007[37]) or communities with seriously limited food supplies beneath ice shelves will disappear at the regional level if food supplies deteriorate further.
A shift in the pelagic realm from a retention-system (only few sinking microorganisms reach the bottom) to a loss-system (much organic material sinks rapidly to the bottom, e.g. in the form of faecal pellets (see Peinert et al., 1989[38])), caused by possible geographic shifts of large krill populations reaching the shelves (Atkinson et al., 2004[39]), could also affect the benthos. How would the benthos respond? Because little is known about the food preferences of key benthic species (Orejas et al., 2003[40]), this question can only be answered in general terms. Suspension feeders that prefer small food particles will suffer from a food overload that would benefit opportunists and those preferring larger particles. Deposit feeders might benefit more from an increase in the amount of food, but less from a change in food quality. Dense concentrations of deep-sea holothurians adapted to generally low-food supplies are typical of the Antarctic shelf (Gutt and Piepenburg, 1991[41]). There is no reason to suppose that their food supplies will dramatically increase with warming of the ocean. The supply of food from the surface may change if increased UV-B radiation alters the composition of the plankton or causes there to be a change in the rate at which planktonic remains settle through the water column. If the ozone hole closes over the next 50 years the effect of UV-B radiation will diminish. Many of the predators target specific food items. That may make them very sensitive to changes in food availability, unless they can adapt their preferences rapidly.
Meiobenthic population structure and density seem to be regulated by variability in food input more than by changes in temperature. Vanhove et al. (2000[42]) investigated a shallow bay at Signy Island (South Orkneys) over an 18 month period and found significant changes in faunal structure. The virtual lack of a feeding break during winter when the bay was covered by ice suggested that food was never limiting. This observation, in combination with the substantial selectivity of the metazoan meiobenthos for specific components of the sedimenting organic matter, such as ice algae or flagellates (Moens et al., 2007[43]), suggests that changing food input in terms of cyclicity, quality and quantity has a significant impact on the meiofauna communities in the shallow waters of the Antarctic.
Acidification
About half of the anthropogenically emitted CO2 has been absorbed by the global ocean (Key et al., 2004[6]), making the global surface ocean more acid by on average 0.1 pH units. This seems like a small amount, but it is equivalent to a 30% increase in hydrogen ions because the pH scale is logarithmic. In both ecological and evolutionary contexts this constitutes a major disturbance. Orr et al. (2005[44]) demonstrate that the rate of acidification will likely increase over the next few centuries, and that acidification will penetrate progressively deeper into the ocean for centuries after fossil fuels have been used up; surface ocean pH levels may become more acid by 0.2 to 0.3 units by 2100. Thus the magnitude of acidification will increase as an agent of disturbance both in absolute terms and spatially. Because levels and rates of acidification are greatest at the surface, organisms in the shallows and on the continental shelf will be influenced first and more seriously – this is likely to be a major problem for animals that secrete CaCO3 skeletons. Nevertheless it should be borne in mind that pre-industrial ocean pH was around 8.2, which is slightly alkaline compared with that of neutral water (pH 7). So the current changes to pH levels of 8.1 and towards 8.0 or 7.9 could be said to be making the ocean less alkaline rather than actually acid (acids have pH<7).
As discussed in The Southern Ocean carbon cycle response to increased CO2 uptake, under normal conditions calcite and aragonite are stable in surface waters because the carbonate ion is supersaturated there. As pH falls, so does the concentration of the carbonate ion. If it falls to the point that carbonate becomes under-saturated, then structures made of calcite or aragonite may begin to dissolve. Because the Southern Ocean has low saturation levels of CaCO3, and because it is the location of much of the uptake of carbon dioxide from the atmosphere, it is at more risk than other areas of approaching under-saturation, particularly for animals that use the aragonite form of CaCO3. Model projections of changing oceanic pH show that Southern Ocean saturation levels could become critical for organisms using aragonite within the next 100 years (Orr et al., 2005[44]). Increased difficulty in synthesising skeletal material could pose major problems for the survival of many Southern Ocean species, particularly the aragonitic pteropods that are an important part of the plankton at the base of the food chain. Despite these fears there is evidence from the North Atlantic of increases in calcification of coccolithophores (calcitic plankton) over the past 200 years at the same time that the ocean has become less alkaline (Iglesias-Rodriguez et al., 2008[45]). Bearing this conflicting evidence in mind, the jury remains out for the moment as far as the current effects of ocean acidification are concerned, though there is a worry about what the effect of the further predicted pH changes may be.
Deoxygenation
Antarctic Bottom Water (AABW) is the principal carrier of oxygen to the global deep sea environment. Very cold, dense and O2 rich surface water sinks at various locations around Antarctica, especially the Weddell Sea, and flows into the deep Southern, Atlantic, Indian and Pacific Oceans. If the surface water is warmed it will become less dense, reducing the flow of surface water to the deep sea. A slower overall flow implies a decline in the flow of oxygen to the deep sea, which will be exacerbated because warm water dissolves less oxygen than cold water. A substantial reduction in the flow of oxygen to the deep sea could have a considerable effect on the global marine biota, given that 50% of the ocean is deep sea floor, which forms the planet’s largest habitat. The biota of the deep sea are so poorly known that it is difficult to gauge the significance of deoxygenation in terms of loss of biomass, abundance, or species richness. That the deep sea biota of the Southern Ocean is very rich and contains species not found elsewhere is evident from the huge number of species new to science caught by recent cruises in the Weddell Sea (Brandt et al., 2007[34]).
To date, the levels of warming detected in the Southern Ocean are too low to be significant in terms of oxygenation. Even if the Southern Ocean does warm significantly over the next 100 years it seems unlikely that the warming per se will be enough to cause significant deoxygenation. Instead, the warming of upper layers could stratify the water column more strongly and so reduce the amounts and rates of sinking, thereby reducing the transport of O2 to the seabed.
References
- ↑ 1.0 1.1 Gutt, J. and Starmans, A. 2001. Quantification of iceberg impact and benthic recolonisation patterns in the Weddell Sea (Antarctica), Polar Biol., 24, 615-619.
- ↑ 2.0 2.1 2.2 Smale, D.A., Barnes, D.K.A. and Fraser, K.P.P. 2007. The influence of depth, site exposure and season on the intensity of iceberg scouring in nearshore Antarctic waters, Polar Biol., 30, 769-779.
- ↑ 3.0 3.1 3.2 Gutt, J. and Piepenburg, D. 2003. Scale-dependent impact on diversity of Antarctic benthos caused by grounding of icebergs, Mar. Ecol. Prog. Ser., 253, 77-83
- ↑ 4.0 4.1 4.2 Cook, A., Fox, A., Vaughan, D. and Ferrigno, J. 2005, Retreating glacier fronts on the Antarctic Peninsula over the past half-century, Science, 308, 541-544.
- ↑ 5.0 5.1 Zwally, H.J, Comiso, J.C., Parkinson, C.L., Cavalieri, D.J. and Gloersen, P. 2002a. Variability of Antarctic sea ice 1979-1998, Journal of Geophysical Research, 10, 3041, doi: 3010.1029/2000JC000733.
- ↑ 6.0 6.1 Key, R.M., Kozyr, A., Sabinec, L., Lee, K., Wanninkhof, R., Bullister, J., Feely, R.A., Millero, F., Mordy, C. and Peng, T-H. 2004. A global ocean carbon climatology: results from GLODAP, Global Biogeochemical Cycles, 18, GB4031
- ↑ Peck, L.S., Brockington, S., Vanhove, S. and Beghyn, M. 1999. Community recovery following catastrophic iceberg impacts in a soft-sediment shallow-water site at Signy Island, Antarctica, Mar. Ecol. Prog. Ser., 186, 1-8.
- ↑ Lee, H.J., Gerdes, D., Vanhove, S. and Vincx, M. 2001a. Meiofauna response to iceberg disturbance on the Antarctic continental shelf at Kapp Norvegia (Weddell Sea), Polar Biol., 24 (12), 926-933.
- ↑ Lee, H.J., Vanhove, S., Peck, L.S. and Vincx, M. 2001b. Recolonisation of meiofauna after catastrophic iceberg scouring in shallow Antarctic sediments, Polar Biol., 24 (12), 918-925.
- ↑ Cowan, E.A., Brachfeld, S.A., Powell, R.D. and Schoolfield, S.C. 2006. Terrane-specific rock magnetic characteristics preserved in glacimarine sediment from southern coastal Alaska, Canadian J. Earth Sci., 43(9), 1269-1282.
- ↑ Gilbert, R., Nielsen, N., Moller, H., Desloges, J.R. and Rasch, M. 2002. Glacimarine sedimentation in Kangerdluk (Disko Fjord) West Greenland, in response to a surging glacier, Mar. Geol., 191, 1-18.
- ↑ Syvitski, J.P.M., Farrow, G.E., Atkinson, R.J.A., Moore, P.G. and Andrews, J.T. 1989. Baffin Island macrobenthos: bottom communities and environmental significance, Arctic, 42, 232-247.
- ↑ Gambi, M.C. and Bussotti, S. 1999. Composition, abundance and stratification of soft-bottom macrobenthos from selected areas of the Ross Sea shelf (Antarctica). Polar Biol 21: 347-354
- ↑ Stockton, W.L. 1984. The biology and ecology of the epifaunal scallop Adamussium colbecki on the west side of McMurdo Sound, Antarctica, Mar. Biol., 78, 171-178.
- ↑ Wiencke, C., Clayton, M. N., Gómez, I., Iken, K., Lüder, U. H., Amsler, C. D., Karsten, U., Hanelt, D., Bischof, K. and Dunton, K. 2007. Life strategy, ecophysiology and ecology of seaweeds in polar waters, Reviews in Environmental Science and Biotechnology, 6(1/3), 95-126.
- ↑ Jacobs, S.S., Giulivi, C.F. and Mele, P.A. 2002. Freshening of the Ross Sea during the late 20th century. Science, 297(5580), 386-389, doi:10.1126/science.1069574.
- ↑ Dayton, P.K. 1989. Interdecadal variation in an Antarctic sponge and its predators from Oceanographic climate shifts, Science, 245, 1484-1486
- ↑ Gutt et al. 2008. The Expedition ANTARKTIS-XXIII/8 of the Research Vessel “Polarstern” in 2006/2007. Ber. Polarforsch. Meeresforsch. 569, 152 pp.
- ↑ 19.0 19.1 Johst, K., Gutt, J., Wissel, C. and Grimm, V. 2006. Diversity and disturbances in the Antarctic megabenthos: feasible versus theoretical disturbance ranges, Ecosystems, 9, 1145-1155.
- ↑ Potthoff, M., Johst, K. and Gutt, J. 2006. How to survive as a pioneer species in the Antarctic benthos: minimum dispersal distance as a function of lifetime and disturbance, Polar Biol., 29, 543-551.
- ↑ Scambos, T.A., Bohlander, J., Shuman, C. and Skvarca, P. 2004. Glacier acceleration and thinner after ice shelf collapse in the Larsen B embayment, Antarctica, Geophys. Res. Lett., 31, doi:10.1029/2004GL020670.
- ↑ Dowdeswell, J.A. and Bamber, J.L. 2007. Keel depths of modem Antarctic icebergs and implications for sea-floor scouring in the geological record, Marine Geology, 243 (1-4), 120-131.
- ↑ Hansen, J., Sato, M., Ruedy, R., Lo, K., Lea, D.W. and Medina-Elizade, M. 2006. Global temperature change, Proc. Natl. Acad. Sci., 103, 14288-14293.
- ↑ Whitehouse, M.J., Meredith, M.P., Rothery, P., Atkinson, A., Ward, P. and Korb, R.E. 2008. Rapid warming of the ocean at South Georgia, Southern Ocean during the 20th Century: forcings, characteristics and implications for lower trophic levels, Deep-Sea Research I, 55, 1218-1228.
- ↑ Meredith, M.P. and King, J.C. 2005. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century, Geophys. Res. Lett., 32, L19604. (doi: 10.1029/2005GL024042)
- ↑ Böning, C. W., A. Dispert, M. Visbeck, S. R. Rintoul, and F. Schwarzkopf, 2008. Observed multi-decadal ocean warming and density trends across the Antarctic Circumpolar Current, Submitted.
- ↑ Poulin, E., Palma, A.T. and Feral, J.-P. 2002. Evolutionary versus ecological success in Antarctic benthic invertebrates, Trends Ecol. Evol., 17, 218-222.
- ↑ Barnes, D.K.A., Fuentes, V., Clarke, A., Schloss, I.R. and Wallace, M.I. 2006b. Spatial and temporal variation in shallow seawater temperature around Antarctica, Deep-Sea Res. II, 53, 853-865.
- ↑ Thrush, S., Dayton, P., Cattaneo-Vietti, R., Chiantore, M., Cummings, V., Andrew, N., Hawes, I., Kim, S., Kvitel, R. and Schwarz, A-M. 2006. Broad-scale factors influencing the biodiversity of coastal benthic communities of the Ross Sea, Deep-Sea Res II, 53, 959-971.
- ↑ Gutt, J. 2000. Some “driving forces” structuring communities of the sublittoral Antarctic macrobenthos, Antarctic Sci., 12 (3), 297-313.
- ↑ Smith, P.J., Steinke, D., McVeagh, S.M., Stewart, A.L., Struthers, C.D. and Roberts, C.D. 2008. Molecular analysis of Southern Ocean skates (Bathyraja) reaveals a new species of Antarctic skate, Journal of Fish Biology, 73, 1170-1182.
- ↑ Billett, D.S.M., Bett, B.J., Rice, A.L., Thurston, M.H., Galeron, J., Sibuet, M. and Wolff, G.A. 2001. Long-term change in the megabenthos of the Porcupine Abyssal Plain (NE Atlantic), Progress in Oceanography, 50, 325-348.
- ↑ Schewe, I. and Soltwedel, T. 2003. Benthic response to ice-edge-induced particle flux in the Arctic Ocean, Polar Biol., 26, 610-620.
- ↑ 34.0 34.1 Brandt, A., Gooday, A.J., ET AL. 2007. First insights into the biodiversity and biogeography of the Southern Ocean deep sea, Nature, 447(7142), 307-311.
- ↑ Post, L.A., Hemer, M.A., O’Brien, P.E., Roberts, D. and Craven, M. 2007. History of benthic colonization beneath the Amery Ice Shelf, East Antarctica, Mar. Ecol. Prog. Ser., 344, 29-37.
- ↑ Riddle, M.J., Craven, M., Goldsworthy, P.M. and Carsey, F. 2007. A diverse benthic assemblage 100 km from open water under the Amery Ice Shelf, Antarctic, Palaeoceanography, 22, PA1204; DIO: 10.1029/2006PA001327.
- ↑ Seiler, J. and Gutt, J. 2007. Can dead sponges still talk?, Antarctic Sci., 19 (3), 337-338.
- ↑ Peinert, R., Von Bodungen, B. and Smetacek, V.S. 1989. Food web structure and loss rate. In Productivity in the ocean: present and past (Eds: Berger WH, Smetacek VS, Wefer G) Wiley-Interscience, Chichester, 35-48.
- ↑ Atkinson, A., Pakhomov, E., Rothery, P., Siegel, V. 2004. Long-term decline in krill stocks and increase in salps within the Southern Ocean, Nature, 432 (7013), 100-103.
- ↑ Orejas, C., Gili, J-M. and Arntz, W. 2003. Role of small-plankton communities in the diet of two Antarctic octocorals (Primnoisis antarctica and Primnoella sp.), Mar. Ecol. Prog. Ser., 250, 105-116.
- ↑ Gutt, J. and Piepenburg, D. 1991. Dense aggregations of three deep-sea holothurians in the southern Weddell Sea, Antarctica, Mar. Ecol. Prog. Ser., 68, 277-285.
- ↑ Vanhove, S., Beghyn, M., Van Gansbeke, D., Bullough, L.W. and Vincx, M. 2000. A seasonally varying biotope at Signy Island, Antarctic: implications for meiofaunal structure, Mar. Ecol. Prog. Ser., 202, 13-25.
- ↑ Moens, T., Vanhove, S., De Mesel, I., Kelemen, B., Janssens, T., Dewicke, A. and Vanreusel, A. 2007. Carbon sources of Antarctic nematodes as revealed by natural carbon isotope ratios and a pulse-chase experiment, Polar Biol., 31 (1), 1-13.
- ↑ 44.0 44.1 Orr, J.C., Fabry, V.J., Aumont, O., Bopp, L., Doney, S.C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R.M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R.G., Plattner, G.K., Rodgers, K.B., Sabine, C.L., Sarmiento, J.L., Schlitzer, R., Slater, R.D., Totterdell, I.J., Weirig, M.F., Yamanaka, Y. and Yool, A. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms, Nature, 437, 681-686.
- ↑ Iglesias-Rodriguez, M.D., Halloran, P.R., Rickaby, R.E.M., Hall, I.R., Colmenero-Hidalgo, E., Gittins, J.R., Green, D.R.H., Tyrrell, T., Gibbs, S.J., Von Dassow, P., Rehm, E., Armbrust, E.V. and Boessenkool, K.P. 2008. Phytoplankton Calcification in a High-CO2 World. Science, 320, 336-340. doi:10.1126/science.1154122.