Marine biota of the Antarctic
- This page is part of the topic Biota of the Antarctic
With the opening of the Drake Passage, separation of Antarctica from South America was complete. This event allowed the inception of the ACC and the establishment of the Polar Front, which acts as an efficient barrier for migration in both directions. Antarctica is an island continent, with the dominant fauna inhabiting the sub-zero water rather than the ice-covered landmass. Unlike other large marine ecosystems, the waters of the continental shelf around Antarctica resemble a closed basin, isolated from other shelf areas in the Southern Hemisphere by distance, current patterns and sub-zero water temperatures. As these isolating conditions developed over the past 30-40 million years, the marine fauna became adapted to a new shelf habitat and their ranges became highly circumscribed. Rates of endemism reach 97% in the case of some marine groups. The attention of evolutionary biologists is drawn to these isolated habitats because of their unusual faunas, which make the waters of the Antarctic shelf comparable to classic evolutionary sites such as the Galapagos.
Over the past 30-40 million years, the physico-chemical features of the Antarctic marine environment experienced a slow and discontinuous transition from the warm-water system of the early Tertiary (15°C) to the cold-water system of today (-1.87°C). As it separated from the other southern continents during the fragmentation of Gondwanaland and moved into its present polar location, Antarctica played a key role in altering ocean circulation and forcing the global climate toward cooling and glaciation. During that time there has been a nearly complete replacement on the Antarctic shelf of the diverse, cosmopolitan temperate fish fauna from the late Eocene by the highly endemic, cold adapted modern fauna. The Antarctic continental shelf offers several striking examples of faunal change and radiation, with fish and some invertebrate groups among the best studied.
Contents
Benthos and demersal fish
The Antarctic continental shelf is very deep in comparison with other continents, reaching 800 m in places, depressed by the weight on the continent of the massive ice sheet, and with troughs reaching to 1,000 m. More than 95% of the shelf is at depths outside the reaches of sea ice keels scouring the seafloor, wave action, scuba divers and sunlight or the photosynthetically active radiation (PAR) that illuminates the euphotic zone. Some 33% of the Antarctica’s continental shelf is covered by floating ice shelves, the largely inaccessible areas below them belonging to the least known habitats on Earth.
The benthic marine fauna of Antarctica is comparatively well known thanks to historical surveys, modern international scientific initiatives such as European Polarstern Study (EPOS), Ecology of the Antarctic Sea Ice Zone (EASIZ), Evolution in the Antarctic (EVOLANTA), Census of Antarctic Marine Life (CAML), Latitudinal Gradient Project (LGP), Food for Benthos on the Antarctic Continental Shelf (FOODBANCS), and individual national projects, although at the regional scale many large gaps in survey data remain (e.g. Griffiths et al., 2009[1]). This fauna currently comprises over 4,000 described species (White 1984[2]; Arntz et al., 1997[3]; Clarke and Johnston, 2003[4]), although it has been estimated that the total macrofauna of the continental shelf may exceed 17,000 species (Gutt et al. 2004[5]). Recent faunistic expeditions, especially those sampling the relatively unknown Antarctic deep-sea (Brandt et al., 2007[6]), reveal tens to hundreds of putative new but so far undescribed species.
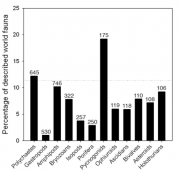
The modern Antarctic shelf-inhabiting benthic fauna is very much an epifauna of sessile filter and particle feeders associated with poorly sorted glacial substrates. In most systematic groups the fauna is highly endemic, eurybathic and possibly stenothermal. Figure 1.14 shows, contrary to an old paradigm, that species richness is quite high; the percentage of the Antarctic species richness as a proportion of global diversity is not much below Antarctica’s percentage of the world’s continental shelf. Two groups, the Antarctic polychaetes and pycnogonids, exceed this value and consequently, have an above global average species richness.
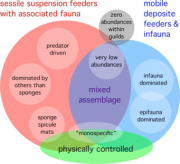
At its most abundant, Antarctica’s benthic fauna is typified by dense, stratified communities of sponges, anemones, ascidians, gorgonians, hydroids, corals, bryozoans, cirripedes, crinoids and dedrochirote holothurians that often form a three-dimensional biogenic architecture below the zone of anchor ice formation and scour by the keels of sea ice (Figure 1.15; Arntz et al., 1994[10], 1997[3]; Gutt, 2000[11]). Associated with these sessile forms is a wandering fauna of ophiuroids, asteroids, echinoids, pycnogonids, isopods, amphipods, shrimps, nemerteans and gastropods. Aside from the dominant sessile suspension feeders we find assemblages in which the fauna benefits from deposited phytodetritus; these organisms include the filter feeding infauna, such as bivalves, or the vagrant deposit feeders, such as elasipode holothurians or ophiuroids (Figure 1.16, Gutt, 2007[9]). Benthic communities cover a full range from an extremely high biomass of several kg wet-weight per m2 to extremely low biomass, abundances and metabolic processes below ice shelves (Azam et al., 1979[12]). Regional and local patchiness can be high due to differences in environmental conditions, especially food supply by currents, biological interactions, disturbance and maybe even incomplete recolonization after the past glaciation. Despite peculiarities in the fauna west of the Antarctic Peninsula, it seems that at coarse spatial resolution there can be said to be a circumpolar fauna typical of the Antarctic.
One of the most important features of the Antarctic benthic fauna is the lack of the durophagous (skeleton-breaking) predation that is a common characteristic of shallow waters elsewhere. Crabs, lobsters and sharks are essentially absent, and there is only a very limited diversity of ray-finned fish (teleosts) and skates (Aronson and Blake, 2001[13]). The benthic fish fauna of Antarctica is no less remarkable in its taxonomic balance. With the exception of a small number of rays known from deeper waters around South Georgia, there are no selachians in the Southern Ocean. Furthermore, many teleost groups are almost completely absent and the fauna is dominated by striking radiations in two groups: the notothenioids, principally on the continental shelf, and the liparids in the deeper waters of the continental slope (Eastman, 1993[14], Eastman and Clarke, 1998[15]).
The perciform suborder Notothenioidei, mostly confined within Antarctic and sub-Antarctic waters, is the dominant component of the Southern Ocean fauna. Indirect indications suggest that notothenioids appeared in the early Tertiary (Eastman, 1993[14]) and began to diversify on the Antarctic continental shelf in the middle Tertiary, gradually adapting to the progressive cooling.
Densities and biomass of the Antarctic meiobenthos (45 µm to 1 mm) are often higher than in other parts of the world (Ingels et al., 2006[16]; Gutzmann et al., 2004[17]; Sebastian et al., 2007[18]). In shelf and slope sediments, nematode communities are generally very diverse at genus level (Vanhove et al., 1999[19]), while the abyssal diversity is comparable with other oceans (Sebastian et al., 2007[18]). In contrast with many macrobenthic taxa (larger than 1 mm), no endemic nematode genera have been identified yet. While there may be no endemic nematode genera, there are endemic nematode species. Information at species level was until recently limited to some scarce taxonomical descriptions. In recent years high local and regional species richness has been revealed in a number of selected dominant nematode genera. Several species found in the Antarctic do show a wide geographical distribution, but most species investigated were new to science (Vermeeren et al., 2004[20]; Fonseca et al., 2006[21]; Ingels et al., 2006[16]; De Mesel et al., 2007[22]).
The present number of macroalgae described from Antarctica is 120 species (Wiencke and Clayton, 2002[23]). Due to the remoteness of the continent and due to incomplete collecting this number is certainly an underestimate. Species numbers are highest in red algae (75) followed by brown and green algae (26 and 17 species, respectively), whilst golden-brown algae are only represented by one species. The proportion of species being endemic to the Antarctic region is highest among the brown and golden-brown algae (Heterokontophyta) (44%), and lowest among the green algae (18%).
Microorganisms
Studies of marine microbial diversity of the Southern Ocean are increasing (Murray and Grzymski, 2007[24]). Approaches have both targeted cultivable heterotrophic bacteria (e.g. Michaud et al. 2004[25]) and used molecular biological approaches. In the genome of the marine psichrophile Pseudoalteromonas haloplanktis TAC125, three genes encoding monomeric hemoglobins and one encoding a flavo-hemoglobin have been discovered (Giordano et al., 2007[26]; Verde et al., In Press). This multiplicity is very rare; their physiological role is under investigation, and may be linked to cold adaptation. From a global ocean perspective, the microbial diversity is moderate and depends on the timing and location of sampling. Broadly, the phylogenetic composition of the microorganisms living in the open water and in sea ice is similar to that found in Arctic Ocean studies and is spread among the different classes of the Proteobacteria (in which Alphaproteobacteria and Gamaproteobacteria are most dominant), Bacteriodetes, and the Archaeal domain. Both Crenarchaeota and Euryarchaeota have been detected in numerous studies (DeLong et al., 1994[27], Murray et al., 1998[28], Church et al., 2003[29], Bano et al., 2004[30]). Both activities and abundance (Pearce, 2008[31]) as well as diversity vary significantly seasonally (Murray et al., 1998[28], Murray and Grzymski, 2007[24]) in coastal waters off the Ingrid Christensen Coast (Prydz Bay, East Antarctica) and Antarctic Peninsula respectively. Summertime samples are dominated by Alphaproteobacteria in the Scotia Sea (Topping et al. 2006[32]), whereas in regions influenced by sea ice melt in the Ross Sea Gammaproteobacteria and Bacteriodetes dominated (Gentile et al., 2006[33]). The extent of diversity in the polar winter is less well known. New directions of research aim to describe microbial diversity associated with Antarctic marine invertebrates (Webster et al., 2004[34], Webster and Bourne, 2007[35], Riesenfeld et al., 2008[36]), and the genome sequences of both cultivated isolates and environmental genome fragments (Grzymski et al., 2006[37]) in oceanic and sea ice communities.
The pelagic system
The open ocean ecosystem of the Southern Ocean is well known for its latitudinal zonation. Three different large-scale subsystems coincide with the distribution of water masses and the ice cover (Hempel, 1985[38]; Nichol and Allison, 1997[39]; Arrigo, 1998[40]; Brierley and Thomas, 2002[41]; Thomas and Dieckmann, 2002[42], Arrigo and Thomas, 2004[43]).
The northern zone occupies the ice-free part of the oceanic West Wind Drift, which is relatively poor in biomass and production. Primary production seems to be limited not by nutrients but by the trace element iron. Despite its High Nutrient – Low Chlorophyll (HNLC) character, there is abundant zooplankton biomass, especially near the Antarctic Convergence and decreasing towards lower latitudes within the West Wind Drift. Zooplankton is dominated by copepods, gelatinous salps, small euphausiids - relatives of the Antarctic krill (Euphausia superba), chaetognaths and amphipods, with copepods constituting more than 60% in most regions and seasons (e.g. Voronina, 1966[44]; Hopkins, 1971[45]). Krill is mostly absent except for the area around South Georgia. Typical representatives of the apex predators are fur seals as well as king and macaroni penguins.
The intermediate zone is the seasonal pack-ice zone, which is typically ice-covered in winter/spring and ice-free in summer/autumn. This zone includes most of the East Wind Drift, the northern branch of the Weddell Gyre, and the Antarctic Peninsula. Large parts of the East Wind Drift are relatively shallow, and this region is the most productive in the Southern Ocean. The Antarctic krill is the dominant species and its summer distribution coincides with that of the sea ice cover in winter (Hempel, 1985[38]; Nicol et al., 2008[46]). The main feature is the coincidence of largest krill concentrations with mixing of water masses of different origins or with sharp changes in bottom topography. Thus, the highest concentrations of krill are found at the shelf slope break in the Bransfield Strait, and in the vicinity of fronts and eddies like the zones of the Antarctic Divergence and the Weddell-Scotia Confluence. Krill can occur in high densities of up to 30,000 individuals per m3 close to the ice edge (e.g. Brierley et al., 2002[47]), where they feed on a great variety of organisms ranging from phytoplankton (particularly diatoms) to larger zooplankton such as copepods (Smetacek and Nicol, 2005[48]). However, recent studies observed krill also in high numbers at depths of up to 3,500 m, probably feeding on abundant phytodetritus (Clarke and Tyler, 2008[49]). In winter, krill have been observed underneath the sea ice exploiting the sea ice algal standing stock (Ross et al., 1996[50]; Daly, 2004[51]). Krill itself provides an important food source for higher trophic levels, including notothenioid fish, seabirds, chinstrap and rockhopper penguins, elephant- fur- and crabeater-seals and the large baleen whales, and hence plays a pivotal role within the Antarctic food web.
The southern zone of the Antarctic open ocean ecosystem is the permanent pack-ice zone and comprises the cold ice shelf water along the continental shelf, particularly in the shallow parts of the inner Weddell and Ross Seas (Hempel, 1985[38]). Phytoplankton production is limited to a short but intensive season, but there is a much larger season of ice algae production. The zooplankton abundance and biomass is low. Small neritic euphausiid species, Euphausia crystallorphias, and juveniles of the pelagic silverfish Pleuragramma antarcticum are typical species of this zone (Hempel, 1985[38]; Boysen-Ennen and Piatkowski, 1988[52]). As is typical for polar systems, the copepod community is characterised by the dominance of only a few species. In general, copepods are a very diverse taxon within the Antarctic zooplankton, accounting for a total of more than 100 species (Schnack-Schiel et al., 1998[53], 2008[54]).
Higher Predators
The permanent pack-ice zone represents the habitat for a highly confined community of seabirds, the most unvarying of any seabird assemblage in the Southern Hemisphere (Ribic and Ainley, 1988[55]). It is composed of Adélie and Emperor penguins, Snow and Antarctic petrels, with the addition during the summer of South Polar skua and Wilson’s storm-petrel. Included as well, and unique to the Antarctic pack-ice zone, are four species of seals: Crabeater, Weddell, Leopard and Ross; and, among cetaceans, the Antarctic minke whale, the ecotype-C of the killer whale (Erickson et al., 1971[56], Laws, 1977a[57],b[58]; LeDuc et al., 2008[59]) and a number of rare toothed whales. These species are in most cases immensely abundant, within their respective groups. A large number of sub-Antarctic species of birds, pinnipeds and cetaceans occur in northern ice-free waters of the Southern Ocean, and move south in the summer as the pack ice recedes.
Species-specific adaptation to low temperature
Most Antarctic marine species are highly stenothermal, with the vast majority having experimental upper lethal temperatures between 5°C and 10°C (Somero and DeVries, 1967[60]; Peck and Conway, 2000[61]). The most stenothermal can only survive in a temperature window between -2°C and +4°C (Peck 1989[62], Pörtner et al., 1999[63]). The physiological processes setting the temperature tolerance limits, at least in marine ectotherms, are associated with reductions in whole animal aerobic scope (Pörtner et al., 1998[64], 1999[63]; Peck et al., 2002[65]). Recently, Pörtner (2002[66]) has elucidated the physiological basis of temperature limits at different levels, and shown a hierarchy of tolerance from the molecular to whole animal. This showed that the tightest limits were set at the whole animal level, with progressively wider tolerance at each step down the physiological hierarchy, and he argued that, in general, adding organismal complexity reduces thermal tolerance. Thus the physiological processes evident in response to varying temperature, at least in acute to medium-term experiments, are a progressive reduction in aerobic scope to a point where it is lost completely and tissues transfer to anaerobic metabolism, the critical physiological limit of Pörtner et al. (1998[64]), and this may have a basis in mitochondrial function (Pörtner et al., 2007[67]). Beyond this point survival is dictated by organismal tolerance to anaerobiosis.
In longer-term studies several Antarctic fish species have been shown to be able to acclimate to 4°C, but not above (Gonzalez-Cabrera et al., 1995[68]; Lowe and Davison, 2005[69]; Seebacher et al., 2005[70]; Podrabsky and Somero, 2006[71]; Jin and DeVries, 2006[72]). Invertebrates, however, appear less able to acclimate to elevated temperatures, as attempts to acclimate animals to temperatures above 2°C failed for the scallop Adamussium colbecki (Bailey et al., 2005[73]). In long-term temperature elevation trials the brachiopod Liothyrella uva survived at 3.0°C but failed at 4.5°C (Peck, 1989[62]) and the bivalve Limopsis marionensis failed at 4°C (Pörtner et al., 1999[63]). Attempts to acclimate the clam Laternula elliptica (S. Morley, pers. comm.) and the brittle star Ophioniotus victoriae (M. Clark pers. comm.) to 3°C have also failed.
The important criteria for population or species survival in a given area is not, however, dictated directly by its physiological tolerance limits, but by the ecophysiological constraints on ability to perform critical biological functions such as feeding, locomotion and reproduction, and how changes in these characters affect ecological balances. Recent investigations of activity in a range of Antarctic marine herbivores have indicated a surprising sensitivity to temperature and a progressive decline in capability consistent with declining aerobic scope (Peck et al., 2004[74]). The large infaunal bivalve mollusc L. elliptica has an experimental upper lethal temperature of 9°C and transfers to anaerobic metabolism at around 6°C (Peck et al., 2002[65]). However, it ceases to rebury after removal from sediment at 5°C, and 50% of the population lose this ability when temperatures reach 2.5°C (Peck et al., 2007[75]). Likewise the limpet N. concinna has an upper lethal temperature of 9.5°C (Peck 1989[62]), but 50% of the population loses the ability to right themselves when turned over at around 2°C, and the scallop A. colbecki dies at 5-6°C, but loses the ability to swim between 1°C and 2°C. These are all major activities that involve extensive muscular activity. The most eurythermal Antarctic marine benthic species identified to date is the starfish Odontaster validus, that survives in raised temperature experiments to 15°C, is capable of performing activity (righting itself when turned over) to 9.5°C, and continues to feed normally and complete a full digestive cycle (Specific Dynamic Action of feeding, SDA, Peck (1998)) to 6°C (Peck et al., In Press). However, in interpreting these studies there remains a considerable gap between the longest experimental periods achievable (with the slowest rates of imposed temperature change), and the rates of change experienced over either evolutionary or contemporary climate change timescales. Thus there are a number of marine invertebrates present on both the Antarctic Peninsula and sub-Antarctic South Georgia, even though the thermal regime of South Georgia is above the experimental limits determined for some of these species living around the Peninsula. Clearly, experimental approaches cannot yet replicate the ability to adapt to climate achievable through evolutionary processes.
References
- ↑ Griffiths, H.J., Barnes, D.K.A. and Linse, K. 2009. Towards a generalized biogeography of the Southern Ocean benthos, Journal of Biogeography, 36, 162-177.
- ↑ White, M.G. 1984. Marine benthos. In: Laws, R.M. (ed.) Antarctic Ecology. Vol. 2. Academic Press, London, 421-461.
- ↑ 3.0 3.1 Arntz, W.E., Gutt, J. and Klages, M. 1997. Antarctic marine biodiversity: an overview. In: Battaglia, B. (ed) Antarctic communities: species, structure and survival. Cambridge University Press, 3-14.
- ↑ Clarke, A. and Johnston, N.M. 2003. Antarctic marine benthic diversity, Oceanogr. Mar. Biol. Ann. Rev., 41, 47-114.
- ↑ Gutt, J., Sirenko, B.I., Smirnov, I.S. and Arntz, W.E. 2004. How many macrobenthic species might inhabit the Antarctic shelf? Antarct. Sci., 16, 11-16.
- ↑ Brandt, A., Gooday, A.J., ET AL. 2007. First insights into the biodiversity and biogeography of the Southern Ocean deep sea, Nature, 447(7142), 307-311.
- ↑ Aronson, R., Thatje S., Clarke A., Peck L.S., Blake D.B., Wilga C.D., Seibel B.A. 2007. Climate change and invisibility of the Antarctic benthos, Annual Review of Ecological and Evolutionary Systems, 38, 129-154.
- ↑ De Broyer, C., Lowry, J.K., Jazdzewski, K. and Robert, H. 2007. Census of marine Life. Synopsis of the Amphipoda of the Southern Ocean. Vol. 1: Part 1. Catalogue of the Gammaridean and Corophiidean Amphipoda (Crustacea) of the Southern Ocean with distribution and ecological data. Institut Royal des Sciences Naturelles de Belgique, Bruxelles, 325 pp.
- ↑ 9.0 9.1 Gutt, J. 2007. Antarctic macro-zpoobenthic communities: a review and an ecological classification, Antarctic Sci., 109 (2), 165-182.
- ↑ Arntz, W.E., Brey, T. and Gallardo, V.A. 1994. Antarctic zoobenthos, Oceanogr. Mar. Biol. Ann. Rev., 32, 241-304.
- ↑ Gutt, J. 2000. Some “driving forces” structuring communities of the sublittoral Antarctic macrobenthos, Antarctic Sci., 12 (3), 297-313.
- ↑ Azam, F., Beers, J.R., Campbell, L., Carlucci, A.F., Holm-Hansen, O., Reis, F.M.H. and Karl, D.M. 1979. Occurrence and metabolic activity of organisms under the Ross Ice Shelf, Antarctica, at station J9, Science, 203, 451-453.
- ↑ Aronson, R.B. and Blake, D.B. 2001. Global Climate Change and the Origin of Modern Benthic Communities in Antarctica, American Zoologist, 41, 27-39.
- ↑ 14.0 14.1 Eastman, J.T. 1993. Antarctic Fish Biology. Evolution in a Unique Environment. Academic Press, San Diego, USA, 322 pp.
- ↑ Eastman, J.T. and Clarke, A. 1998. A comparison of adaptive radiations of antarctic fish with those of nonantarctic fish. In: Prisco G. di, Pisano, E., Clarke, A. (eds) Fishes of Antarctica. Springer, Milan, 3-26.
- ↑ 16.0 16.1 Ingels, J., Vanhove, S., De Mesel, I. and Vanreusel, A. 2006. The biodiversity and biogeography of the free-living nematode genera Desmodora and Desmodorella (family Desmodoridae) at both sides of the Scotia Arc, Polar Biol., 29 (11), 936-949.
- ↑ Gutzmann, E., Martínez Arbizu, P., Rose, A. and Veit-Köhler, G. 2004. Meiofauna communities along an abyssal depth gradient in the in the Drake Passage, Deep-Sea Res .II, 51 (14-16), 1617-1628.
- ↑ 18.0 18.1 Sebastian, S., Raes, M., De Mesel, I. and Vanreusel, A. 2007. Comparison of the nematode fauna from the Weddell Sea Abyssal Plain with two North Atlantic abyssal sites, Deep-Sea Res. II, 54, 1727-1736.
- ↑ Vanhove, S., Arntz, W. and Vincx, M. 1999. Comparative study of the nematode communities on the southeastern Weddell Sea shelf and slope (Antarctica), Mar. Ecol. Prog. Ser., 181, 237-256
- ↑ Vermeeren, H., Vanreusel, A. and Vanhove, S. 2004. Species distribution within the free-living marine nematode genus Dichromadora in the Weddell Sea and adjacent areas, Deep-Sea Res. II, 51 (14-16), 1643-1664.
- ↑ Fonseca, G., Vanreusel, A. and Decraemer, W. 2006. Taxonomy and biogeography of Molgolaimus Ditlevsen, 1921 (Nematoda : Chromadoria) with reference to the origins of deep sea nematodes, Antarct. Sci., 18 (1), 23-50.
- ↑ De Mesel, I., Lee, H.J., Vanhove, S., Vincx, M., Vanreusel, A. 2007. Species diversity and distribution within the deep-sea nematode genus Acantholaimus on the continental shelf and slope in Antarctica, Polar Biol., 29 (10), 860-871.
- ↑ Wiencke, C. and Clayton, M.N. 2002. Antarctic Seaweeds, (Synopses of the Antarctic Benthos. Ed.: J. W. Wägele and J. Sieg ; Vol. 9), Ruggell : Gantner ; Königstein : Koeltz Scientific Books., 239 pp.
- ↑ 24.0 24.1 Murray, A.E. and, Grzymski, J.J. 2007. Diversity and genomics of Antarctic marine micro-organisms, Philosophical Transactions of the Royal Society B-Biological Sciences, 362, 2259-2271.
- ↑ Michaud, L., Cello, F.D., Brilli, M., Fani, R., Guidice, A.L. and Bruni, V. 2004. Biodiversity of cultivable psychrotrophic marine bacteria isolated from Terra Nova Bay (Ross Sea, Antarctica), FEMS Microbiol. Lett., 230, 63-71.
- ↑ Giordano, D., Parrilli, E., Dettaï, A., Russo, R., Barbiero, G., Marino, G., Lecointre, G., Di Prisco, G., Tutino, L. and Verde, C. 2007. The truncated hemoglobins in the Antarctic psychrophilic bacterium Pseudoalteromonas haloplanktis TAC125, Gene, 398, 143-155.
- ↑ Delong, E.F., Wu, K.Y., Prezelin, B.B. and Jovine, R.V.M. 1994. High abundance of Archaea in Antarctic marine picoplankton, Nature, 371, 695-697
- ↑ 28.0 28.1 Murray, A.E., Preston, C.M., Massana, R., Taylor, L.T., Blakis, A., Wu, K. and Delong, E.F. 1998. Seasonal and spatial variability of bacterial and archaeal assemblages in the coastal waters off Anvers Island, Antarctica, Appl. Environ. Microbiol., 64, 2585-2595.
- ↑ Church, M.J., Delong, E.F., Ducklow, H.W., Karner, M.B., Preston, C.M. and Karl, D.M. 2003. Abundance and distribution of planktonic Archaea and Bacteria in the waters west of the Antarctic Peninsula, Limnology and Oceanography, 48, 1893-1902.
- ↑ Bano, N., Ruffin, S., Ransom, B. and Hollibaugh, J. T. 2004. Phylogenetic composition of Arctic Ocean archaeal assemblages and comparison with antarctic assemblages, Appl. Environ. Microbiol, 70, 781-789.
- ↑ Pearce, D. 2008. Biodiversity of the bacterioplankton in the surface waters around Southern Thule in the Southern Ocean, Antarct. Sci., 20, 291-300.
- ↑ Topping, J.N., Heywood, J.L., Ward, P. and Zubkov, M.V. 2006. Bacterioplankton composition in the Scotia Sea, Antarctica, during the austral summer of 2003, Aquat. Microbial Ecol., 45, 229-235.
- ↑ Gentile, G., Giuliano, L., D'auria, G., Smedile, F., Azzaro, M., De Domenico, M. and Yakimov, M.M. 2006. Study of bacterial communities in Antarctic coastal waters by a combination of 16S rRNA and 16S rDNA sequencing, Environ. Microbiol., 8, 2150-2161.
- ↑ Webster, N.S., Negri, A.P., Munro, M. and Battershill, C.N. 2004. Diverse microbial communities inhabit Antarctic sponges, Environ. Microbiol., 6, 288-300.
- ↑ Webster, N.S. and Bourne, D. 2007. Bacterial community structure associated with the Antarctic soft coral, Alcyonium antarcticum, Fems. Microbiol. Ecol., 59, 81-94.
- ↑ Riesenfeld, C.S., Murray, A.E. and Baker, B.J. 2008. Characterization of the microbial community and polyketide biosynthetic potential in the Palmerolide-producing tunicate, Synoicum adareanum., J. Nat. Prod., 71, 1812-1818.
- ↑ Grzymski, J.J., Carter, B.J., Delong, E.F., Feldman, R.A., Ghadiri, A. and Murray, A. E. 2006. Comparative genomics of DNA fragments from six antarctic marine planktonic bacteria, Appl. Environ. Microbiol., 72, 1532-1541.
- ↑ 38.0 38.1 38.2 38.3 Hempel, G. 1985. On the biology of polar seas, particularly the Southern Ocean. In: Marine Biology of Polar Regions and Effects of Stress on Marine Organisms (Gray JS, Christiansen ME, eds). John Wiley and Sons Ltd., Chichester, 3-33.
- ↑ Nichol, S.A. and, Allison, I. 1997. The frozen skin of the Southern Ocean, American Scientist, 85, 426-439
- ↑ Arrigo, K.R. 1998. Antarctic sea ice: biological processes, interactions and variability. ed; Lizotte, Michael P.; Lizotte, Michael P.; Arrigo, Kevin R., Antarctic Research Series, 73, 1-198.
- ↑ Brierley, A.S. and Thomas, D.N. 2002. On the ecology of Southern Ocean pack ice. Advances in Marine Biology, 43, 171-278.
- ↑ Thomas, D.N. and Dieckmann, G.S. 2002. Antarctic sea ice-a habitat for extremophiles, Science, 295, 641-644.
- ↑ Arrigo, K.R. and Thomas, D.N. 2004. Large-scale importance of sea ice biology in the Southern Ocean, Antarctic Science 16, 471-486, doi:10.1017/S0954102004002263.
- ↑ Voronina, N.M. 1966. Distribution of the zooplankton biomass in the Southern Ocean, Oceanology, 6, 836-846.
- ↑ Hopkins, T.L. 1971 Zooplankton standing crop in the Pacific sector of the Antarctic. In Biology of the Antarctic Seas IV, (G.A. Llano, I.E. Wallen, eds.), Antarctic Research Series, 17, 347-362.
- ↑ Nicol, S., Worby, A. and Leaper, R. 2008. Changes in the sea-ice ecosystem: potential effects on krill and baleen whales, Marine and Freshwater Research, 59, 361-382.
- ↑ Brierley, A.S., Fernandes, P.G., Brandon, M.A., Armstrong, F., Millard, McPhail, S.D., Stevenson, P., Pebody, M., Perrett, J., Squires, M., Bone, D.G. and Griffiths, G. 2002. Antarctic Krill under sea ice: elevated abundance in a narrow band just south of ice edge, Science, 295, 1890-1892.
- ↑ Smetacek, V. and Nicol, S. 2005. Polar ocean ecosystems in a changing world, Nature, 437, 362-368.
- ↑ Clarke, A. and, Tyler, P.A. 2008. Adult antarctic krill feeding at abyssal depths, Current Biology, 18, 282-285.
- ↑ Ross, R.M., Quetin, L.B. and Lascara, C. 1996. Distribution of Antarctic krill and dominant zooplankton west of the Antarctic Peninsula. In: Foundations for Ecological Research West of the Antarctic Peninsula, (Eds. R.M. Ross, E.E. Hofmann and L.B. Quetin), American Geophysical Union, Washington, D.C. Antarctic Research Series, 70, 199-217
- ↑ Daly, K.L. 2004. Overwintering growth and development of larval Euphausia superba: an interannual comparison under varying environmental conditions west of the Antarctic Peninsula, Deep-Sea Research II, 51, 2139-2168.
- ↑ Boysen-Ennen, E. and Piatkowski, U. 1988. Meso- and macrozooplankton communities in the Weddell Sea, Polar Biol., 9, 17-35.
- ↑ Schnack-Schiel, S.B., Hagen, W. and Mizdalski, E. 1998. Seasonal carbon dynamics of Antarctic copepods in the Weddell Sea, Journal of Marine Systems, 17, 305-311.
- ↑ Schnack-Schiel, S.B., Michels, J., Mizdalski, E., Schodlok, M. and Schröder, M. 2008. Composition and community structure of zooplankton in the sea ice covered western Weddell Sea in spring 2004 – with emphasis on calanoid copepods, Deep-Sea Research II, 55, 1040-1055.
- ↑ Ribic C.A. and Ainley, D.G. 1988. Constancy of seabird species assemblages: an exploratory look, Biol. Oceanogr., 6, 175-202.
- ↑ Erickson, A.W., Siniff, D.B., Cline, D.R. and Hofman, R.J. 1971. Distributional ecology of Antarctic seals. In Symposium on Antarctic Ice and Water Masses: (G. Deacon, ed.), 55-76. SCAR, Cambridge.
- ↑ Laws, R. 1977a. Seals and whales of the Southern Ocean, Phil. Trans Royal Soc. London. B279, 81-96.
- ↑ Laws, R.M. 1977b. The significance of vertebrates in the Antarctic marine ecosystem. In: Adaptations within Antarctic Ecosystem (ed. G.A. Llano). Gulf Publishing Company, Houston, 411-438.
- ↑ Leduc, R.G., Roberston, K.M. and Pitman, R.L. 2008. Mitochondrial sequence divergence among Antarctic killer whale ecotypes is consistent with multiple species, Biology Letters, 4, 426-429.
- ↑ Somero, G.N. and Devries, A.L. 1967. Temperature tolerance of some Antarctic fishes, Science, 156, 257-258.
- ↑ Peck, L.S. and Conway, L.Z. 2000. The myth of metabolic cold adaptation: oxygen consumption in stenothermal Antarctic bivalves. In Harper E, Tatlor JD, Crame JA (eds). Evolutionary biology of the bivalvia, 177. Geol Soc Special Publications, London, 441-450.
- ↑ 62.0 62.1 62.2 Peck, L.S. 1989. Temperature and basal metabolism in two Antarctic marine herbivores, J. Exp. Mar. Biol. Ecol., 127, 1-12.
- ↑ 63.0 63.1 63.2 Pörtner, H.O., Peck, L., Zielinski, S. and Conway, L.Z. 1999. Intracellular pH and energy metabolism in the highly stenothermal Antarctic bivalve Limopsis marionensis as a function of ambient temperature, Polar Biology, 22, 17-30.
- ↑ 64.0 64.1 Pörtner, H.O., Hardewig, I., Sartorius, F.J. and Van Dijk, P.L.M. 1998. Energetic aspects of cold adaptation: critical temperatures in metabolic, ionic and acid-base regulation? In Pörtner HO, Playle R (eds) Cold Ocean Physiology, Cambridge University Press, Cambridge, 88-120.
- ↑ 65.0 65.1 Peck, L.S., Pörtner, H.O. and Hardewig, I. 2002. Metabolic demand, oxygen supply, and critical temperatures in the Antarctic bivalve Laternula elliptica, Physiol. Biochem. Zool., 75, 123-133.
- ↑ Pörtner, H.O. 2002. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals, Comp. Biochem. Physiol. A, 132, 739-761.
- ↑ Pörtner, H.O., Peck, L.S. and Somero, G.N. 2007. Thermal limits and adaptation: an integrative view (Antarctic Ecology: From Genes to Ecosystems), Phil. Trans. R. Soc. B, 362, 2233-2258.
- ↑ Gonzalez-Cabrera, J.J., Dowd, F., Pedibhotla, V.K., Rosario, R., Stanley-Samuelson, D. and Petzel, D. 1995. Enhanced hypo-osmoregulation induced bt warm acclimation in Antarctic fish is mediated by increased gill and kidneyNa+/K+-ATPase activities, J. Exp. Biol., 198, 2279-2291.
- ↑ Lowe, C.J. and Davison, W. 2005. Plasma osmolarity, glucose concentration and erythrocyte responses of two Antarctic nototheniid fishes to acute and chronic thermal change, J. Fish Biol., 67(3), 752-766.
- ↑ Seebacher, F., Davison, W., Lowe, C.J. and Franklin, C.E. 2005. A falsification of the thermal specialization paradigm: compensation for elevated temperatures in Antarctic fish, Biol. Lett., 2, 151-154.
- ↑ Podrabsky, J.E. and Somero, G.N. 2006. Inducible heat tolerance in Antarctic notothenioid fishes, Polar Biology, 30, 39-43, DOI 10.1007/s00300-00006-00157-y.
- ↑ Jin, Y. and Devries, A.L. 2006. Antifreeze glycoprotein levels in Antarctic notothenioid fishes inhabiting different thermal environments and the effect of warm acclimation, Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 144, 290-300.
- ↑ Bailey, D.M., Johnston, I.A., Peck, L.S. 2005. Invertebrate muscle performance at high latitude: swimming activity in the Antarctic scallop, Adamussium colbecki, Polar Biol, 28, 464-469.
- ↑ Peck, L.S., Webb, K. and Bailey, D. 2004. Extreme sensitivity of biological function to temperature in Antarctic marine species, Functional Ecology, 18, 625-630.
- ↑ Peck, L.S., Morley, S.A., Pörtner, H.O. and Clark, M.S. 2007. Thermal limits of burrowing capacity are linked to oxygen availability and size in the Antarctic clam Laternula elliptica. Oecologia. Published on line DOI 10.1007/s00442-007-0858-0.
