Atmospheric chemistry in the instrumental period
- This page is part of the topic Antarctic climate and environment change in the instrumental period
Contents
Antarctic stratospheric ozone in the instrumental period
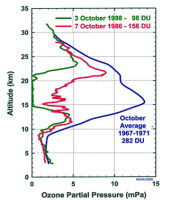
Historically ozone values were around 300 Dobson Units (DU) at the beginning of the winter (March), and similar at the end (August). The pattern began to change in the 1970s, following widespread releases of CFCs and Halons in the atmosphere (see below). Now, at the end of August values are about 10% less than they were in the 1970s, and decrease at about 1% per day to reach about 100 DU in October. Most of this loss occurs between 14 and 22 km altitude within the polar vortex, where virtually all ozone is now destroyed (Figure 4.14). Ozone values substantially recover with the warming in late spring, when the vortex dissipates and air from outside is mixed (Figure 4.15).
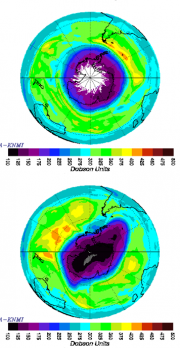
The ozone hole is caused by reactive chlorine and bromine gases, formed from the breakdown products of CFCs and Halons, which were liberated in the troposphere from spray-cans, refrigerators and fire extinguishers. They have a typical stratospheric lifetime of 50 to 100 years. But the development of the ozone hole is also strongly linked to the dynamics of the polar vortex because it acts as a barrier (Figure 4.16, top panel). During winter, lower stratospheric temperatures drop below –80°C, and at these temperatures clouds form despite the dryness of the stratosphere, initially composed of nitric acid trihydrate, but of ice if 5 to 10°C colder. On the cloud surfaces, the degradation products of the CFCs react to form chlorine gas:
- HCl + ClNO3 → HNO3 + Cl2
followed by photo-dissociation to chlorine atoms when sunlit, then reaction with ozone to form highly reactive ClO. Similar reactions take place involving the bromine compounds that result from degradation of halons. The highly reactive chlorine and bromine compounds then take part in a series of photochemical reactions such as:
- Cl + O3 → ClO + O2
- ClO + O3 → Cl + 2O2
in which in effect the Cl acts as a catalyst and gets recycled time and again. The net result of the catalysis is that:
- 2O3 → 3O2
The process is helped by the absence of NO2 (converted to HNO3 and absorbed into the clouds), which would otherwise react with the ClO to recreate ClNO3 and so remove reactive gases from the catalytic cycle.
As the vortex warms the clouds disappear, but the reactive chlorine and bromine compounds continue the ozone depletion for some weeks, until converted back to HCl and HBr. With further warming the vortex begins to break down and the sub-polar ozone-rich air sweeps across the continent.
The ozone hole is often offset from the pole towards the Atlantic, reaching as far north as 50°S. Ozone provides a screen against ultraviolet light of wavelength shorter than about 315 nm (UV-B), which can cause sunburn, cataracts and skin cancer in humans. Hence such an offset poses a serious health threat to the inhabitants of southern South America. It also poses a risk for flora and fauna, as UV-B can damage DNA, and can bleach chlorophyll that then becomes non-functional (see Terrestrial biology in the instrumental period).
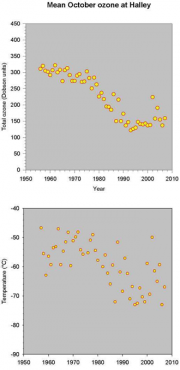
Another effect of the ozone hole is on the temperatures within the vortex, because ozone is a greenhouse gas that absorbs solar radiation. The absence of ozone has therefore resulted in significant reductions in temperature in spring (Figure 4.17), in November reaching a difference of up to 15°C in comparison with pre-ozone hole years.
The Montreal Protocol is an international agreement that has phased out production of CFCs, Halons, and some other organic chlorides and bromides, collectively referred to as Ozone Depleting Substances (ODSs). Because of its success, the amounts of ODSs in the stratosphere are now starting to decrease. However, there is as yet no convincing sign of any reduction in the size or depth of the ozone hole, although the sustained increases up the 1990s have not continued. Recent changes in measures of Antarctic ozone depletion have ranged from little change over the past 10 years (ozone hole area), to some signs of ozone increase (Bodeker et al., 2005[1]). The halt in rapid ozone hole growth can be ascribed to the fact that almost all of the ozone between 12 and 24 km in the core of the vortex is now being destroyed (WMO, 2003[2]), and is therefore comparatively insensitive to small changes in ODS amount.
Antarctic Tropospheric Chemistry
Although often thought of as a single unit, the atmosphere is divided up into a number of regions. These are determined by the temperature gradient with height. The lowest region is referred to as the troposphere. In the troposphere, temperature generally decreases with height until a point is reached where this trend reverses. This upper limit is referred to as the tropopause. The height of the tropopause varies with latitude and is roughly at 8 km above hand or sea in the polar regions. The troposphere itself is nominally subdivided into layers; the lowest is the “boundary layer”, a part of the troposphere that is directly influenced by the surface of the Earth in the exchange of heat, momentum and moisture. The height of the boundary layer is determined by physical constraints such as temperature and wind speed, and over Antarctica can vary considerably from tens to hundreds of metres. Above the boundary layer is the free troposphere, a region remote from the direct influence of the Earth’s surface.
Over many regions of the world, the chemistry of the troposphere is studied in order to understand the effect of emissions from human activities. These might be direct emissions from industrial processes, or emissions associated with, for example, agriculture. Such activities release relatively reactive and short-lived trace gases into the atmosphere, and change it considerably from its natural state. Antarctica supports no major population centres and lies at a considerable distance from anthropogenic emission sources, so although some longer-lived pollutants do reach Antarctica, the Antarctic troposphere is a relatively unperturbed natural background atmosphere.
Compared with many other aspects of Antarctic science, the chemistry of the Antarctic troposphere has received relatively little attention. A primary reason for this was the perception that the chemical composition would be relatively uninteresting, with low concentrations of reactive trace gases like the hydroxyl (OH) or hydroperoxy (HO2) radicals or the nitrogen oxides (NO and NO2), and merely a sluggish chemistry dominated by unreactive reservoir gases that had been transported from distant source regions. Atmospheric chemists interested in studying a clean background atmosphere would naturally choose to work in a location that was more easily accessible and with more benign ambient conditions.
Nevertheless, Antarctica is a significant part of the Earth system, and studies of Antarctic tropospheric chemistry have gradually become a recognised part of the work of national operators. A major driver has been the fact that deep ice cores are drilled from Antarctic ice sheets from which paleo-scientists strive to reconstruct changes in the Earth’s atmospheric composition and climate through time. This work relies on analysing and interpreting changes in impurities held within the ice cores. A correct interpretation relies on knowing how the impurity entered the ice and any associated depositional or post-depositional effects. It would be cavalier to believe we could correctly reconstruct a past atmosphere from these chemical tracers without properly understanding the tropospheric chemistry of the present day.
The early studies of Antarctic tropospheric chemistry focused mainly on aerosols and long-lived radiatively and stratospherically important gases. Aerosols are important components of ice core impurities and can act as valuable proxies for environmental changes through time. For example, sea salt is a prime component of aerosol in coastal Antarctica, and sodium and chloride are both easily measured in ice cores. Studies of sea salt aerosol have been necessary to determine dominant sources and as a fingerprint for the behaviour of marine air masses. They have shown that, whereas for most of the globe the open ocean is the source of sea salt, in the polar regions most sea salt may be generated within and adjacent to the zone of newly-forming sea ice. This suggests that sea salt measured in ice cores might provide a proxy for assessing the extent of sea ice and how this varied under different climatic conditions. The records of long-lived gases have provided invaluable evidence of how the global atmosphere has recently changed. For example, the record of boundary layer carbon dioxide (CO2), which has been measured at South Pole since 1957, has shown the massive rise in this potent greenhouse gas, and importantly, has bridged the gap between ice core records of CO2 and present day ambient measurements. Also, systematic continuous measurements of the atmospheric CO2 concentration at Syowa Station since 1984 revealed clear evidence for a seasonal cycle, a secular trend and interannual variations (Morimoto et al., 2003[3]). The seasonal cycle varied from year to year, with especially large amplitudes in 1992 and 1998 and a large phase delay in 1993. A rapid increase in the CO2 concentration was observed in 1987, 1994 and 1998 in association with ENSO events, and very low increase rate in 1991 to 1993, related to the Pinatubo eruption. From measurements of the stable carbon isotope ratio (δ13C) of atmospheric CO2, it was found that the rapid increase of the CO2 concentration was accompanied by a rapid decrease of δ13C. Considering the fact that the δ13C values of terrestrial biospheric CO2 are lower than those of atmospheric CO2, the interannual variations of the CO2 increase are ascribed primarily to changes of the CO2 exchange between the atmospheric and terrestrial biosphere due to climate change in association with an ENSO event. It has been confirmed that long term continuous observations of greenhouse gases are essential in the Antarctic to monitor climate variations.
To clarify variations at the surface and in the transport of greenhouse gases, it was indispensable to know the vertical distributions of concentrations. Balloon-borne campaigns using a cryogenic sampler with a large balloon were carried out at Syowa Station in 1998 and 2003/04 to examine the vertical distribution of greenhouse gas concentrations up to 30 km in the stratosphere (Aoki et al., 2003[4]). Together with bi-polar balloon-borne observations in the Arctic in 1997 and similar observations over Japan since 1985, stratospheric CO2 above 20 – 25 km showed a secular increase with an average rate of 1.5 ppmv/yr, slightly less than the rate at the surface, and some increase of CO2 age in the stratosphere. The O2/N2 ratio from these stratospheric air samples was analyzed to better understand the global carbon cycle (Ishidoya et al., 2006[5]). The vertical profile of O2/N2 showed a gradual decrease with height in the stratosphere, indicating the gravitational separation of molecules.
Beyond its role as an archive of global change, studies of the Antarctic troposphere have revealed a highly individual and active chemical system that is likely itself in the future to be an active player within a changing climate system. The rest of this section details this chemistry.

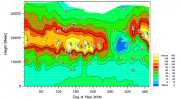
Of the more reactive trace gases, only surface ozone has historically been measured with any vigour. Year-round measurements were made at Halley station as early as 1958, but continuous records began considerably later, in 1975 at South Pole and in the 1980s at McMurdo/Arrival Heights and at Neumayer. Today, surface ozone is measured routinely at four coastal sites (those mentioned above plus Syowa) as well as at Sanae, some 170 km inland, and at South Pole on the Antarctic plateau (Helmig et al., 2007[6]). The records show both interesting similarities and differences (see Figure 4.18). At nearly all stations, surface ozone reaches its maximum concentration during the winter months and is at its minimum during the summer. This is the classic seasonal cycle for a trace gas whose concentration is balanced by increases arising from air mass transport and destruction by the direct action of the Sun or by sunlight-initiated chemistry.
During the Antarctic spring, however, significant differences are evident between the coastal sites and those lying inland. While at South Pole and Sanae, the decline from the winter maximum towards summertime values is essentially smooth, surface ozone at coastal sites exhibits extremely rapid and large episodic losses during the spring months (Wessel et al., 1998[7]; Jones et al., 2006[8]). These ozone depletion events (ODEs) can last for several days and ozone concentrations can drop as low as instrumental detection limits.
This behaviour is natural and occurs at coastal sites in both polar regions. The ozone loss is driven by reactions with halogen atoms, primarily bromine, in chemical cycles analogous to stratospheric ozone depletion. The following reactions were proposed to explain ODEs observed in the Arctic (Barrie et al., 1988[9]):
- 2 x (O3 + Br → BrO + O2)
- BrO + BrO → 2Br + O2
- net: 2 O3 → 3 O2
Key to this process is the fact that bromine is recycled from bromine monoxide (BrO) to bromine atoms (Br) without the production of ozone. Ozone is therefore destroyed in a catalytic cycle whereby the bromine atoms responsible are regenerated and ready to react again with other ozone molecules. Other radicals, such as ClO, IO or HO2 can also be involved in BrO recycling. For a full discussion see the review by Simpson et al. (2007[10]).
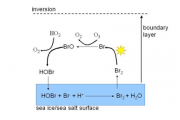
Such recycling of course does not generate “new” halogens, so the important question is – what is the original source of the bromine atoms? Theoretical and laboratory studies have demonstrated that a series of reactions, widely referred to as the “Bromine Explosion” (shown schematically in Figure 4.19), are the source.
Bromide (Br-) is a ubiquitous ion found in sea water, albeit at concentrations ~280 times lower than chloride (Cl-). Bromide derived from sea water reacts with HOBr, a molecule with a gas-phase source, and produces bromine molecules that are then released back into the boundary layer. The action of sunlight splits the bromine molecule into two bromine atoms, and ozone destruction can commence. The process is contained within the boundary layer. This reaction process requires a liquid (or “quasi-liquid”) phase with concentrated sea salt brine, and the exact nature of this is still under debate. There is some evidence that this surface is associated with newly-forming sea ice (Rankin et al., 2002[11]; Jones et al., 2006[8]), and other evidence suggesting that sea salt-laden snow plays a role (McConnell et al., 1992[12]; Simpson et al., 2007[10]). Certainly, sea salt (via sea ice/aerosol etc.) is fundamental as the prime bromide source, and anything that might affect its availability is likely to affect the frequency of ozone depletion events.
The surface ozone records discussed above also reveal a second surprising feature. During the summer months at South Pole, surface ozone concentrations increase even above those measured during winter. This suggests that, rather than experiencing net destruction, ozone is being produced in situ. The only route for this within the troposphere is through photolysis of NO2, but NO2 is normally associated with polluted air and has low concentrations in pristine atmospheres. The questions then arise – what is the source of this ozone, and why, out of all the measurement locations, is it only evident at South Pole?
The answers lie within a new area of atmospheric science; that of snow photochemistry. The traditional view of polar snow was that it had an important influence on albedo, and also that it acted as a cap to exchange of trace gases between the boundary layer and the land or sea surfaces below. However, an active chemical role had not been anticipated. That view has now been overturned, and it has been shown through many chemical studies in the field, in the laboratory, and in modelling calculations, that snow is a major source of reactive trace gases to the polar boundary layer both through physical and photochemical release processes (see review by Grannas et al., 2007[13]). For example, nitrate impurities within snow are photolysed to produce nitrogen oxides (NO and NO2) (Honrath et al., 1999[14]; Jones et al, 2000[15]; Dibb et al., 2002[16]; Beine et al., 2002a[17]) that are released to the overlying boundary layer (Jones et al., 2001[18]; Honrath et al., 2002[19]; Beine et al., 2002b[20]). Although this occurs across the Antarctic snowpack, the effects are particularly noticeable at South Pole because of the characteristically shallow boundary layer at this site. Emissions from snow are concentrated within a confined layer of the atmosphere, which accentuates the resultant chemistry (Davis et al., 2004[21]). NO2 released from snow, therefore, becomes a significant source of local ozone, accounting for the surface ozone measurements described above (Crawford et al., 2001[22]).
The influence of the snowpack on boundary layer chemistry is enormous and, crucially, encompasses fast reactive photochemistry. As well as releasing NOx, the snow is a source of hydrogen peroxide (H2O2), formaldehyde (HCHO) and nitrous acid (HONO). These can be direct sources of OH, a highly reactive radical that reacts with numerous other trace gases thus driving tropospheric chemistry. Furthermore, enhanced concentrations of NO will generate OH through the reaction NO + HO2 → NO2 + OH. As a result of snowpack emissions, the boundary layer above the snow-covered Antarctic is far from being quiescent, but contains a fast and reactive photochemical system.
Recent measurements at Halley station have shown that halogens are also major players in fast reactive photochemistry in the coastal boundary layer. As well as high concentrations of BrO measured during the spring, the seasonal cycle of iodine monoxide (IO) shows an equally high springtime peak, as well as significant concentrations during the summertime (Saiz-Lopez et al., 2007[23]). Indeed, modelling studies based around field measurements have shown that at Halley, although snow photochemistry is active, it is the halogens that control the cycling of reactive radicals and hence the chemical pathways (e.g. Bloss et al., 2007[24]). The origin of IO is not absolutely known, but the proposed source is from diatoms, marine phytoplankton that colonise the underside of sea ice.
The chemistry of the Antarctic troposphere is now known to be extremely complex and unusual. The sunlit months are characterised by fast photochemical systems with chemical origins associated with snow and sea ice. It is precisely this link, between the atmosphere and the cryosphere that makes present day tropospheric chemistry systems vulnerable to a changing climate.
Aerosol, clouds and radiation

Aerosol particles are known to cause significant effects on the radiative budget of the Earth, both directly, through scattering and absorption of shortwave and longwave radiation, and indirectly by acting as condensation nuclei. In polar regions, where the surface albedo can exceed 0.85 in areas covered by snow and ice, aerosols may produce appreciable warming at the surface due to multiple reflection if highly absorbing particles are suspended above these bright surfaces. It is important to determine the radiative properties of particles within the entire atmosphere column to evaluate accurately the radiative forcing. Large sets of radiometer (actinometer, pyrheliometer and sunphotometer) measurements have been carried out over the past 30 years at different Antarctic sites and examined to estimate ensemble average and long-term trends of background aerosol optical depth (AOD at 500 nm; Tomasi et al., 2007[25]) (Figure 4.20). No significant trend was observed, except some large variation during the periods affected by the volcanic eruptions of El Chichon (1982), Pinatubo (1991) and Cerro Hudson (1971). To address topics related to the radiative forcing by polar aerosols in particular, a programme referred to as POLAR-AOD was conducted as a major project of the IPY 2007-2008.
Since the typical example of highly absorbing particles is black carbon (BC), behaviours of BC have been examined at several Antarctic stations. From the wintering intensive observations of aerosols at Syowa Station during 2004 and 2006, unique seasonal variation was obtained with a winter maximum, in contrast to the summer maximum obtained from observations at similar coastal stations as Halley or Neumayer (Wolff and Cachier, 1998[26]), and in parallel to the results at Amsterdam Island (Pereira et al., 2006[27]). Also an interesting pathway of BC to Syowa was found in summer months, a high peak of BC concentration occurred with local katabatic wind maximum following trajectories coming from the continental side. As there would be no source of BC inland, those trajectories must have originated from South America or Africa in response to biomass burning, for example. These pathways were confirmed in the airborne campaign (ANTSYO-II) jointly conducted by AWI, Germany and NIPR, Japan with the AWI aircraft Polar 2, around Neumayer and Syowa stations in the 2006/07 season.
Clouds are an extremely important part of the Antarctic climate system and have a significant influence on the surface radiation balance and hence also on biological processes. Yet there are large uncertainties in quantifying their role, and there is little information on how cloud amount and cloud properties have changed in recent decades. Although the general distribution of clouds is well-known - with large amounts of cloud cover over sea ice or open water in the Southern Ocean, and low cloud amounts over the continent (King and Turner, 1997[28]), we still have no reliable cloud climatology due to deficiencies in deriving cloud amounts from passive satellite sensor data over snow and ice surfaces (Yamanouchi and Kawaguchi, 1992[29]; Kato et al., 2006[30]). Determining trends in cloud amount from the station data is difficult because of jumps when the observers change. However, with careful assessment of the observations some trends have been estimated. The 50 years of observations from Syowa Station revealed a 10% increase in cloud cover over that period (Yamanouchi and Shudou, 2007[31]), although no trend was detected in the observations from South Pole (Town et al., 2007[32]).
The radiation budget is the key aspect of studies of Antarctic climate, and the general features of the surface radiation budget at the stations on the continent have been described (Liljequist, 1956[33]; Kuhn et al., 1977[34]; Yamanouchi, 1983[35]; Yamanouchi and Kawaguchi, 1984[36]). High precision surface radiation budget measurements continue at Neumayer and Syowa stations as part of the global Baseline Surface Radiation Network (BSRN; Ohmura et al., 1998[37]). From the global measurements a decline in solar radiation over the land surface was apparent up to 1990 - a phenomenon known as “global dimming”. Widespread “brightening” has been observed since the late 1980s globally and in the Antarctic (Wild et al., 2003[38]), although there is no consensus yet on the Antarctic measurements (e.g. see Yamanouchi and Shudou, 2007[31]). As for the top-of-atmosphere radiation budget, following the pioneer work by Raschke et al. (1973[39]) using satellite data in the early stage, several measurements have been conducted as part of the Earth Radiation Budget Experiment (ERBE) and Clouds and the Earth’s Radiant Energy System (CERES). As yet there is no clear conclusion about radiation trends at that level in the Antarctic (Yamanouchi and Charlock, 1997[40]; Kato et al., 2006[30]).
Spatial variations in atmospheric chemistry suggested from snow cores
Ice core chemistry includes a broad range of measurements such as: major soluble ions, trace elements, radionuclides, and organic acids. The following is a synthesis of some of the major findings related to the understanding of the chemistry of the atmosphere over Antarctica as understood from the examination of the chemistry of ice cores.
An updated compilation of published and new data of major ion (Ca, Cl, K, Mg, Na, NO3, SO4) and methylsulfonate (MS) concentrations in snow from 520 Antarctic sites is provided by the national ITASE programmes of Australia, Brazil, China, Germany, Italy, Japan, Korea, New Zealand, Norway, United Kingdom, United States of America, and the national Antarctic programme of Finland (Bertler et al., 2005[41]). The comparison shows that snow chemistry concentrations vary by up to four orders of magnitude across Antarctica and exhibit distinct geographical patterns (see example Na in Figure 4.21). This Antarctic-wide comparison provides a unique opportunity to improve our understanding of the fundamental factors that ultimately control the chemistry of a snow or ice sample.
As expected, the East Antarctic interior shows significantly lower values (~2 ppb to ~30 ppb) than the coastal sites (~75 ppb to 14,680 ppb). However, high values have also been reported from Marie Byrd Land at high elevation, and low concentrations in the vicinity of the East Antarctic coastlines (Kaiser Wilhelm Land and Terra Adélie). Furthermore, the change from very low to very high concentrations seems to occur within a narrow band in the vicinity of the coast. While high Na deposition is readily explained in coastal areas due to high sea salt input, the narrow zone of marine air mass intrusions (mesoscale cyclonic activity) coincides with the rapid decrease of Na concentrations in the Antarctic interior. Here the katabatic wind streams, transporting Na-depleted air masses from the interior towards the coast, compete with the Na-rich coastal air masses. In contrast, the Antarctic Peninsula shows overall high values and no trends, caused by strong sea salt input all year round and a secondary non-sea-salt contribution from ice-free mountain peaks. Most of the data points located on the Antarctic Peninsula are surface samples representing winter snow. As Na peaks in most regions of Antarctica during winter, the higher Na concentrations reported from the Antarctic Peninsula are partially caused by this bias. This and similar maps for Ca, Cl, K, Mg, NO3, SO4) and MS are available in Bertler et al. (2005[41]).
References
- ↑ Bodeker, G.E., Shiona, H. and Eskes, H. 2005. Indicators of Antarctic ozone depletion, Atmos. Chem. Phys., 5, 2603-2615.
- ↑ WMO, 2003. Scientific Assessment of Ozone Depletion: 2002, World Meteorological Organisation Global Ozone Research and Monitoring Project - Report No. 47, Geneva, Switzerland (2003).
- ↑ Morimoto, S., Nakazawa, T., Aoki, S., Hashida, G. and Yamanouchi, T. 2003. Concentration variations of atmospheric CO2 observed at Syowa Station, Antarctica from 1984 to 2000, Tellus, 55B, 170-177.
- ↑ Aoki, S., Nakazawa, T., Machida, T., Sugawara, S., Morimoto, S., Hashida, G., Yamanouchi, T., Kawamura, K. and Honda, H. 2003. Carbon Dioxide Variations in the Stratosphere Over Japan, Scandinavia and Antarctic, Tellus, 55B, 178-186.
- ↑ Ishidoya, S., Sugawara, S., Hashida, G., Morimoto, S. Aoki, S., Nakazawa, T. and Yamanouchi, T. 2006. Vertical profiles of the O2/N2 ratio in the stratosphere over Japan and Antarctica, Geophys. Res. Lett., 33, L13701, doi:10.1029/2006GL025886.
- ↑ 6.0 6.1 Helmig, D., Oltmans, S., Carlson, D., Lamarque, J.-F., Jones, A.E., Labuschagne, C., Anlauf, K. and Hayden, K. 2007. A review of surface ozone in the polar regions, Atmos. Env., 41, 5138-5161, doi:10.1016/j.atmosenv.2006.09.053.
- ↑ Wessel, S., Aoki, S., Winkler, P., Weller, R., Herber, A., Gernandt, H. and Schrems, O. 1998. Tropospheric ozone depletion in polar regions: A comparison of observations in the Arctic and Antarctic, Tellus, 50B, 34-50.
- ↑ 8.0 8.1 Jones, A.E., Anderson, P.S., Wolff, E.W., Turner, J., Rankin, A.M. and Colwell, S.R. 2006. A role for newly-forming sea ice in springtime polar tropospheric ozone loss? Observational evidence from Halley station, Antarctica, J. Geophys. Res., 111, D08306, doi:10.1029/2005JD006566.
- ↑ Barrie, L.A., Bottenheim, J.W., Schnell, R.C., Crutzen, P.J. and Rasmussen, R.A. 1988. Ozone destruction and photochemical reactions at polar sunrise in the lower Arctic atmosphere, Nature, 334, 138-141.
- ↑ 10.0 10.1 Simpson, W.R., Von Glasow, R., Riedel, K., Anderson, P. Ariya, P., Bottenheim, J., Burrows, J., Carpenter, L.J., Frieß, U., Goodsite, M.E., Heard, D., Hutterli, H., Jacobi, H.-W., Kaleschke, L., Neff, B., Plane, J., Platt, U., Richter, A., Roscoe, H., Sander, R., Shepson, P., Sodeau, J., Steffen, A., Wagner, T. and Wolff, E. 2007. Halogens and their role in polar boundary-layer ozone depletion, Atmos. Chem. Phys., 7, 4375-4418.
- ↑ Rankin, A.M., Wolff, E.W. and Martin, S. 2002. Frost flowers: Implications for tropospheric chemistry and ice core interpretation, J. Geophys. Res., 107, 4683, doi:10.1029/2002JD002492.
- ↑ McConnell, J.C., Henderson, G.S., Barrie, L., Bottenheim, J., Niki, H., Langford, C.H. and Templeton, E.M.J. 1992. Photochemical bromine production implicated in Arctic boundary-layer ozone depletion, Nature, 355, 150-152.
- ↑ Grannas, A.M. and 34 others. 2007. An overview of snow photochemistry: evidence, mechanisms and impacts, Atmos. Chem. Phys. Discuss., 7, 4165-4283.
- ↑ Honrath, R.E., Peterson, M.C., Guo, S., Dibb, J.E., Shepson, P.B. and Campbell, B. 1999. Evidence of NOx production within or upon ice particles in the Greenland snowpack, Geophys. Res. Lett., 26, 695-698.
- ↑ Jones, V.J., Hodgson, D.A. and Chepstow-Lusty, A. 2000. Palaeolimnological evidence for marked Holocene environmental changes on Signy Island, Antarctica, The Holocene, 10, 43-60.
- ↑ Dibb, J. E., Arsenault, M., Peterson, M.C. and Honrath, R.E. 2002. Fast nitrogen oxide photochemistry in Summit, Greenland snow, Atmos. Environ., 26, 2501-2511.
- ↑ Beine, H.J., Dominé, F., Simpson, W.R., Honrath, R.E., Sparapani, R., Zhou, X. and King, M. 2002a. Snow-pile and chamber experiments during the Polar Sunrise Experiment ‘Alert 2000’: Exploration of nitrogen chemistry, Atmos. Environment, 36(15-16), 2707-2719.
- ↑ Jones, A.E., Weller, R., Anderson, P.S., Jacobi, H.-W., Wolff, E.W., Schrems, O. and Miller, H. 2001. Measurements of NOx emissions from the Antarctic snowpack, Geophys. Res. Lett., 28, 1499-1502.
- ↑ Honrath, R.E., Lu, Y., Peterson, M.C., Dibb, J.E., Arsenault, M.A., Cullen, N.J. and Steffen, K., 2002. Vertical fluxes of NOx, HONO and HNO3 above the snowpack at Summit, Greenland, Atmos. Environ., 36, 2629-2640.
- ↑ Beine, H.J., Honrath, R.E., Dominé, F., Simpson, W.R. and Fuentes, J.D. 2002b. NOx during background and ozone depletion periods at Alert: Fluxes above the snow surface, J. Geophys. Res., 107(D21), 4584, doi:10.1029/2002JD002082.
- ↑ Davis, D., Chen, G., Buhr, M., Crawford, J., Lenschow, D., Lefer, B., Shetter, R., Eisele, F., Mauldin, L. and Hogan, A. 2004. South Pole NOx chemistry: an assessment of factors controlling variability and absolute levels, Atmos. Environ., 38, 5375-5388.
- ↑ Crawford, J.H., Davis, D.D., Chen, G., Buhr, M., Oltmans, S., Weller, R., Mauldin, L., Eisele, F., Shetter, R., Lefer, B., Arimoto, R. and Hogan, A. 2001. Evidence for photochemical production of ozone at the South Pole surface, Geophys. Res. Lett., 28, 3641-3644.
- ↑ Saiz-Lopez, A., Mahajan, A.S., Salmon, R.A., Bauguitte, S.J.-B., Jones, A.E., Roscoe, H.K. and Plane, J.M.C. 2007. Boundary layer halogens in coastal Antarctica, Science, 317, 348-351, DOI: 10.1126/science.1141408.
- ↑ Bloss, W. J., Lee, J.D., Heard, D.E., Salmon, R.A., Bauguitte, S.J.-B., Roscoe, H.K. and Jones, A.E. 2007. Observations of OH and HO2 radicals in coastal Antarctica, Atmos. Chem. Phys., 7, 4171-4185.
- ↑ 25.0 25.1 Tomasi, C. and 30 collaborators. 2007. Aerosols on polar regions: An historical overview on the basis of optical depth and in-situ observations, J. Geophys. Res., 112, D16205, doi: 10.1029/2007JD008432.
- ↑ Wolff, E.W. and Cachier, H. 1998. Concentrations and seasonal cycle of black carbon in aerosol at a coastal Antarctic station, J. Geophys. Res., 103(D9), 11033-11042, 10.1029/97JD01363.
- ↑ Pereira, E.B., Evangelista, H., Pereira, K.C.D., Cavalcanti, I.F.A. and Setzer, A.W. 2006. Apportionment of black carbon in the South Shetland Islands, Antarctic Peninsula, J. Geophys. Res., 111, D03303, doi: 10.1029/2005JD006086.
- ↑ King, J.C. and Turner, J. 1997. Antarctic meteorology and climatology, Cambridge University Press, Cambridge, UK, 409 pp.
- ↑ Yamanouchi, T. and Kawaguchi, S. 1992. Cloud distribution in the Antarctic from a VHRR data and radiation measurements at the surface, International Journal of Remote Sensing, 13, 111-127.
- ↑ 30.0 30.1 Kato, S., Loeb, N.G., Minnis, P., Francis, J.A., Charlock, T.P., Rutan, D.A., Clothiaux, E.E. and Sun-Mack, S. 2006. Seasonal and interannual variations of top-of-atmosphere irradiance and cloud cover over polar regions derived from the CERES data set, Geophys. Res. Lett., 33, L19804, doi: 10.1029/2006GL026685.
- ↑ 31.0 31.1 Yamanouchi, T. and Shudou, Y. 2007. Trends in cloud amount and radiative fluxes at Syowa Station, Antarctica, Polar Science, 1, 17-23.
- ↑ Town, M. S., Walden, V.P. and Warren, S.G. 2007. Cloud cover over the South Pole from visual observations, satellite retrievals, and surface-based infrared measurements, J. Climate, 20, 544-559.
- ↑ Liljequist, G.H. 1956. Energy exchange of an antarctic snow-field: A. Short-wave radiation; B. Long-wave radiation and radiation balance. Norweigian-British-Swedish Antarctic Expedition 1949-1952, Scientific Results, 2, Part 1, Norsk Polarinstitut, Oslo, 1-184.
- ↑ Kuhn, M., Kundla, L.S. and Streschein, L.A. 1977. The radiation budget at Plateau Station, Antarctica, Am. Geophys. Union, 41-73 (Antarctic Res. Ser., 25).
- ↑ Yamanouchi, T. 1983. Variation of incident solar flux and snow albedo on the solar zenith angle and cloud cover, at Mizuho Station, Antarctica. J. Meteor. Soc. Jpn, 61, 879-893.
- ↑ Yamanouchi, T. and Kawaguchi, S. 1984. Longwave radiation balance under a strong surface inversion in the katabatic wind zone, Antarctica. J. Geohys. Res., 89, 11771-11778.
- ↑ Ohmura, A., Gilgen, H., Hegner, H., Muller, G., Wild, M., Dutton, E., Forgan, B., Frohlich, C., Philipona, R., Heimo, A., Konig-Lango, G., McArthur, B., Pinker, R., Whitlock, C.H. and Dehne, K. 1998. Baseline Surface Radiation Network (BSRN/WCRP): New precision radiometery for climate research, Bull. Am. Meteorol. Soc., 10, 2115-2136.
- ↑ Wild, M., Calanca, P., Scherrer, S.C. and Ohmura, A. 2003. Effects of polar ice sheets on global sea level in high-resolution greenhouse scenarios, J. Geophys. Res., 108, 4165, doi:10.1029/2002JD002451.
- ↑ Raschke, E., Vonder Haar, T.H., Bandeen, W.R. and Pasternak, M. 1973. The annual radiation balance of the earth-atmosphere system during 1969-1970 from Nimbus 3 measurements, J. Armos. Sci., 30, 341-364.
- ↑ Yamanouchi, T. and Charlock, T.P. 1997. Effects of clouds, ice sheet and sea ice on the earth radiation budget in the Antarctic, J. Geophys. Res., 102, 6953-6970.
- ↑ 41.0 41.1 Bertler, N.A.N. and 53 others. 2005. Snow Chemistry across Antarctica, Annals of Glaciology, 41, 167-179.